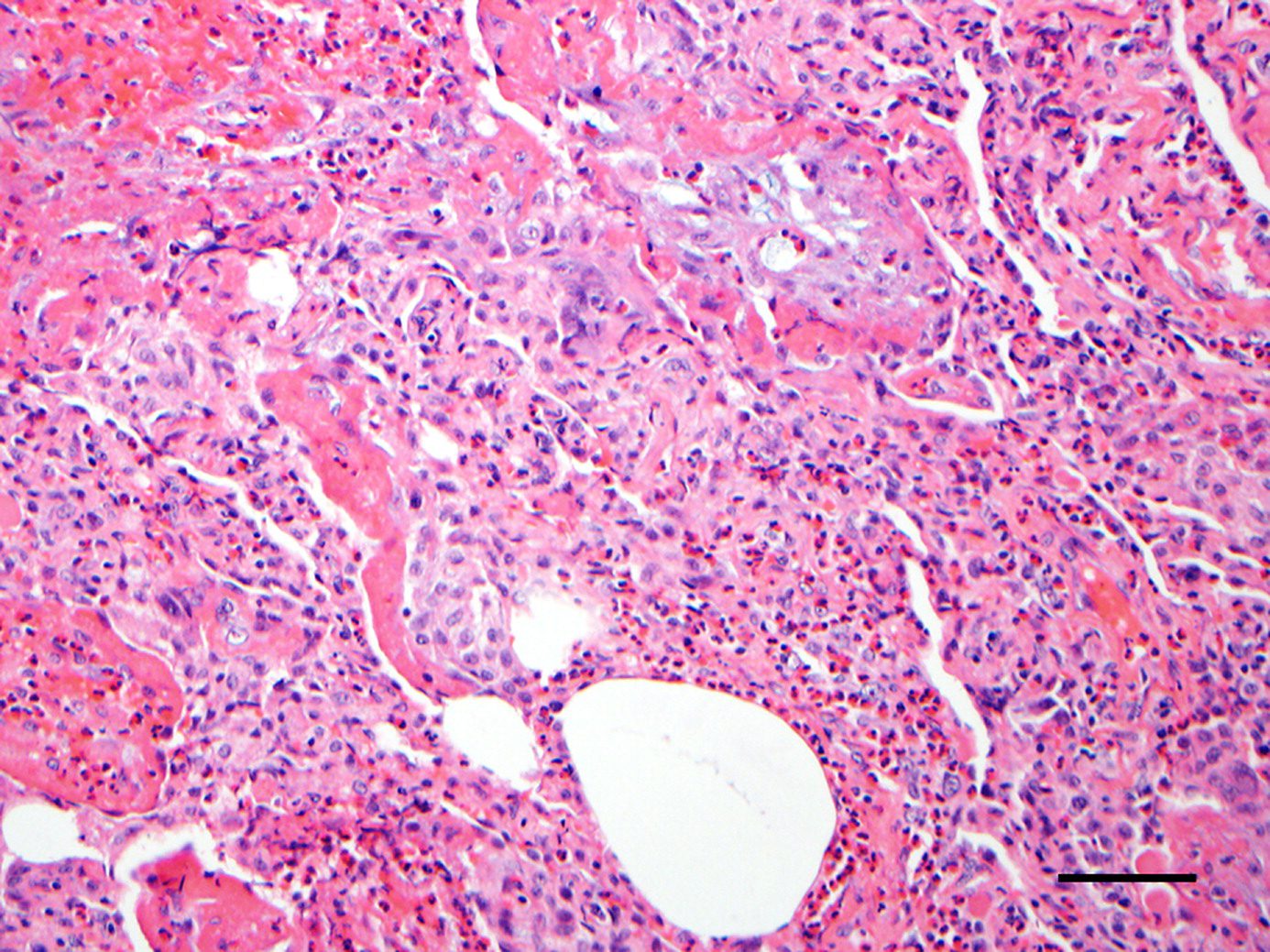
Infiltration of eosinophils in the alveolar spaces, bronchial walls, and, to a lesser extent, in the interstitium. Acute and/or organizing diffuse alveolar damage is present. ,
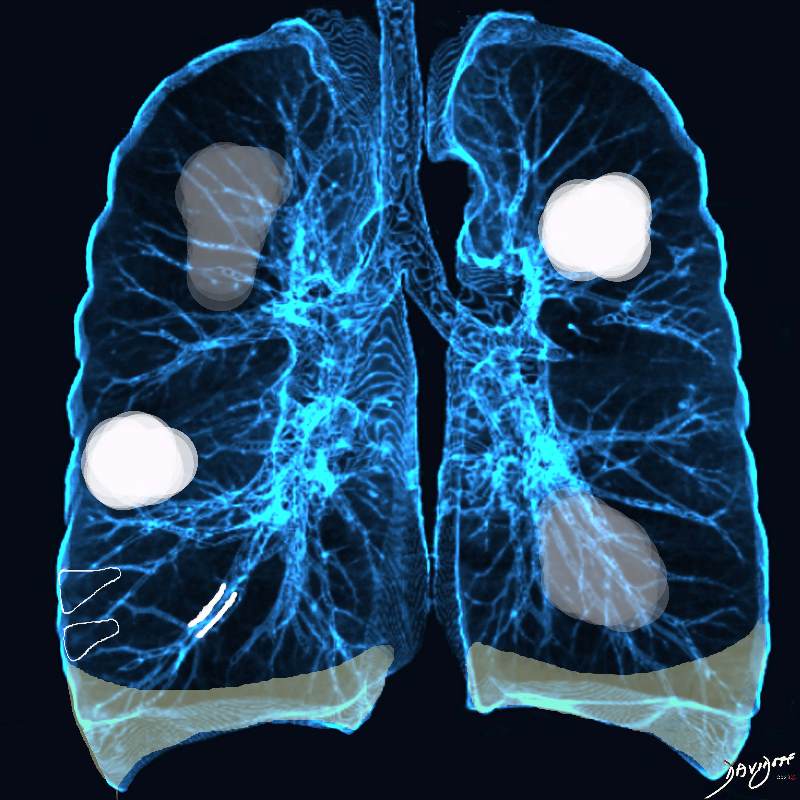
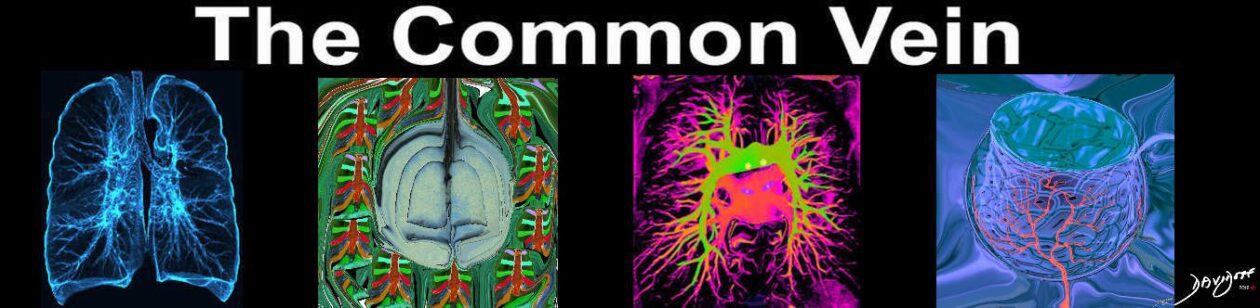
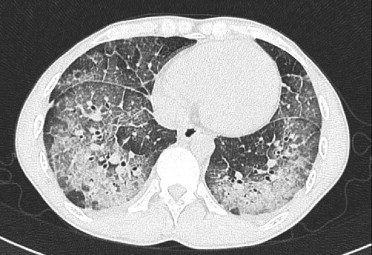
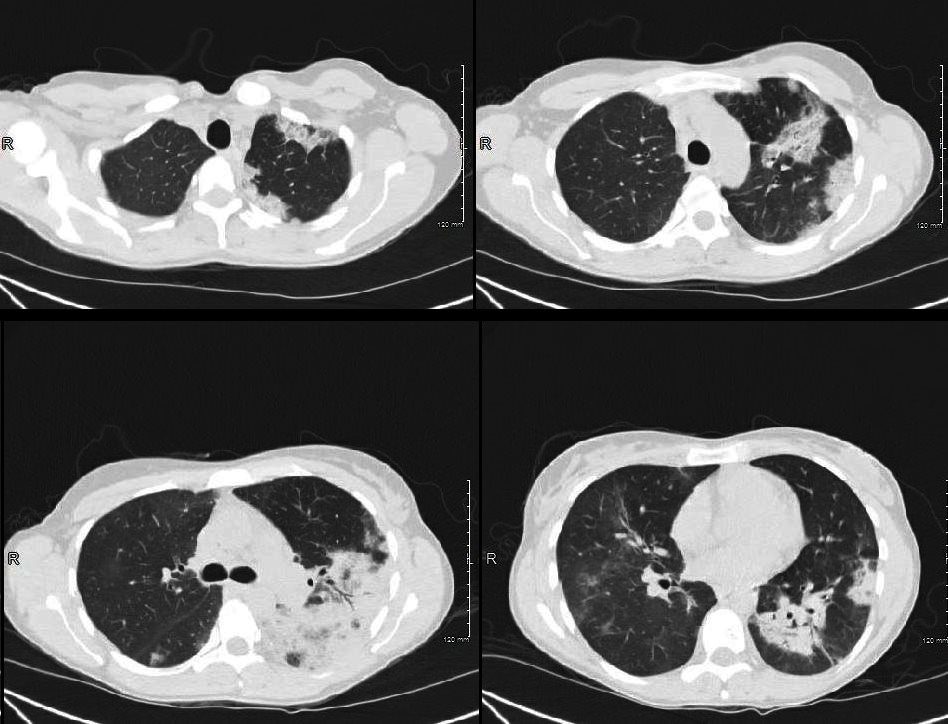
Acute Eosinophilic pneumonia is characterised by ground glass opacities (100%) and sometimes consolidation (55%) most commonly with a random distribution 60%. Septal lines (90%) and thickening of the bronchovascular bundles (66%) and bilateral pleural effusions (75%) were common. Ashley Davidoff MD TheCommonvein.net lungs-0775-b (Reference De Giacomi F et al)
Acute Eosinophilic pneumonia is characterised by ground glass opacities (100%) and sometimes consolidation (55%) most commonly with a random distribution 60%. Septal lines (90%) and thickening of the bronchovascular bundles (66%) and bilateral pleural effusions (75%) were common.
Ashley Davidoff MD TheCommonvein.net lungs-0775-bL (Reference De Giacomi F et al)
By Blausen Medical – BruceBlaus
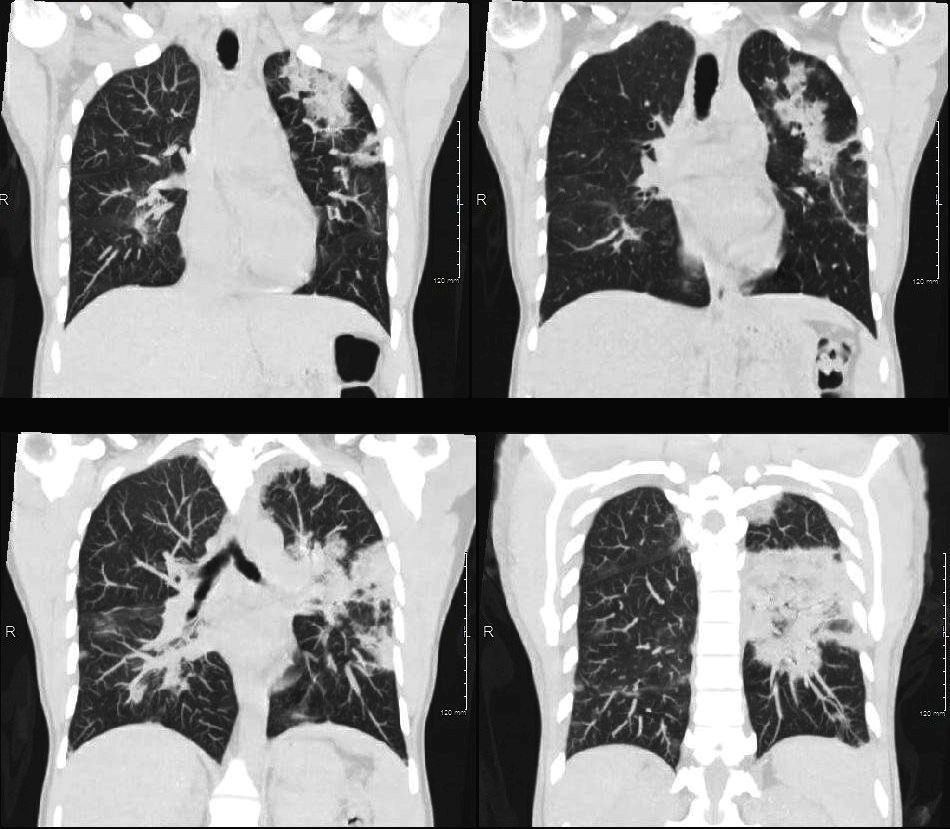
From De Giacomi F et al Acute Eosinophilic Pneumonia. Causes, Diagnosis, and Management American Journal of Respiratory and Critical Care Medicine Volume 197, Issue 6
From De Giacomi F et al Acute Eosinophilic Pneumonia. Causes, Diagnosis, and Management American Journal of Respiratory and Critical Care Medicine Volume 197, Issue 6
- rare disease inflammatory disease of the small airways and alveoli
- characterized by eosinophilic infiltration of the pulmonary parenchyma (alveoli and interstitium)
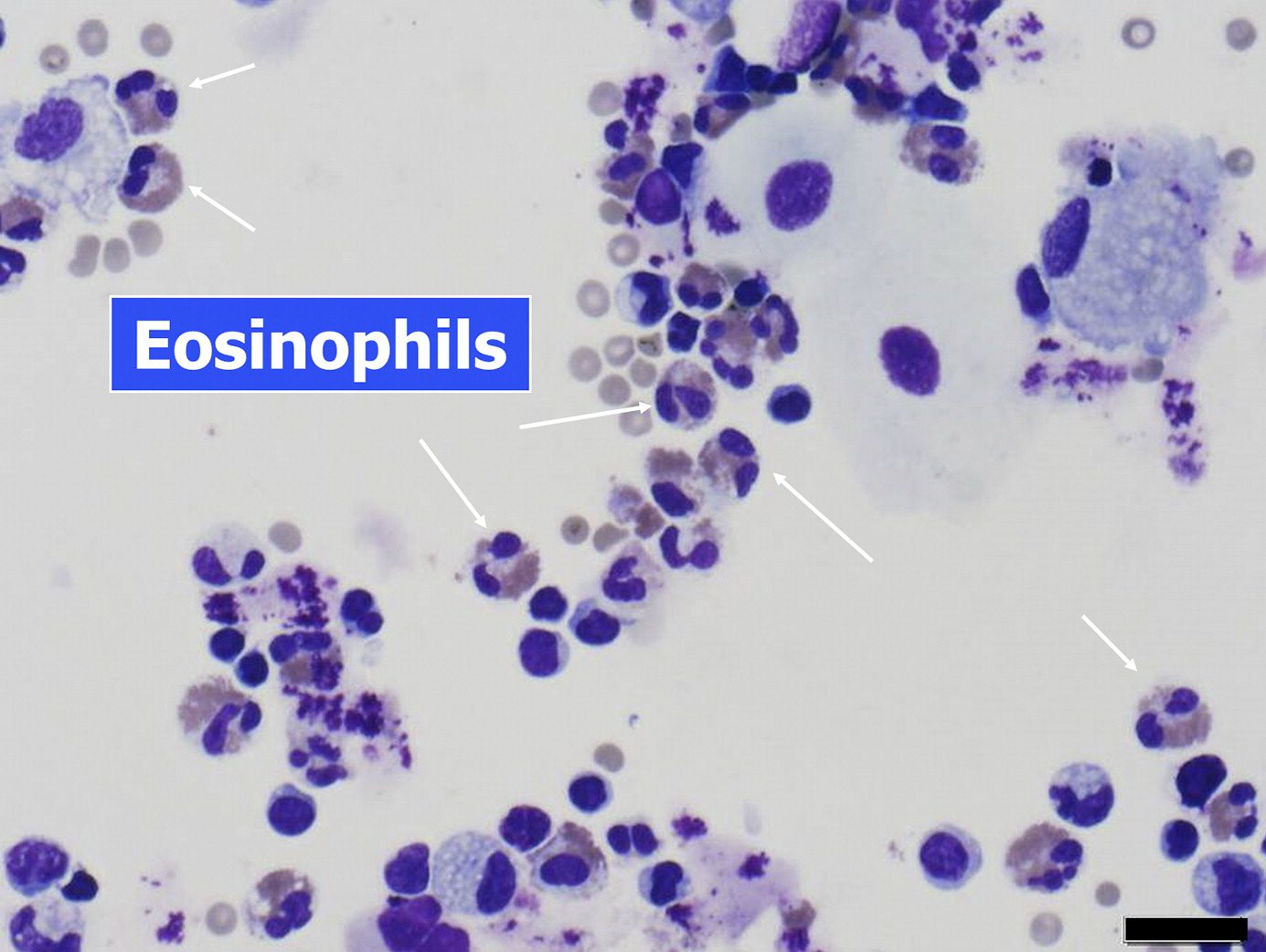
- eosinophil leukocytes
- multifunctional cells for
- innate and adaptive immunity, including
- inflammatory reactions to
- parasitic helminth, bacterial, and viral infections
- inflammatory reactions to
- innate and adaptive immunity, including
- multifunctional cells for
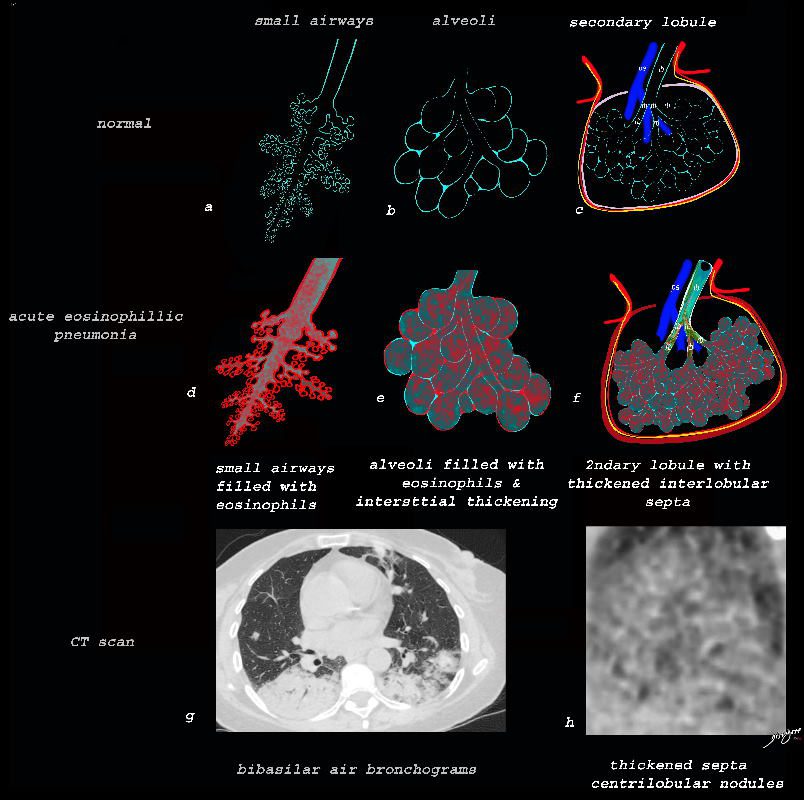
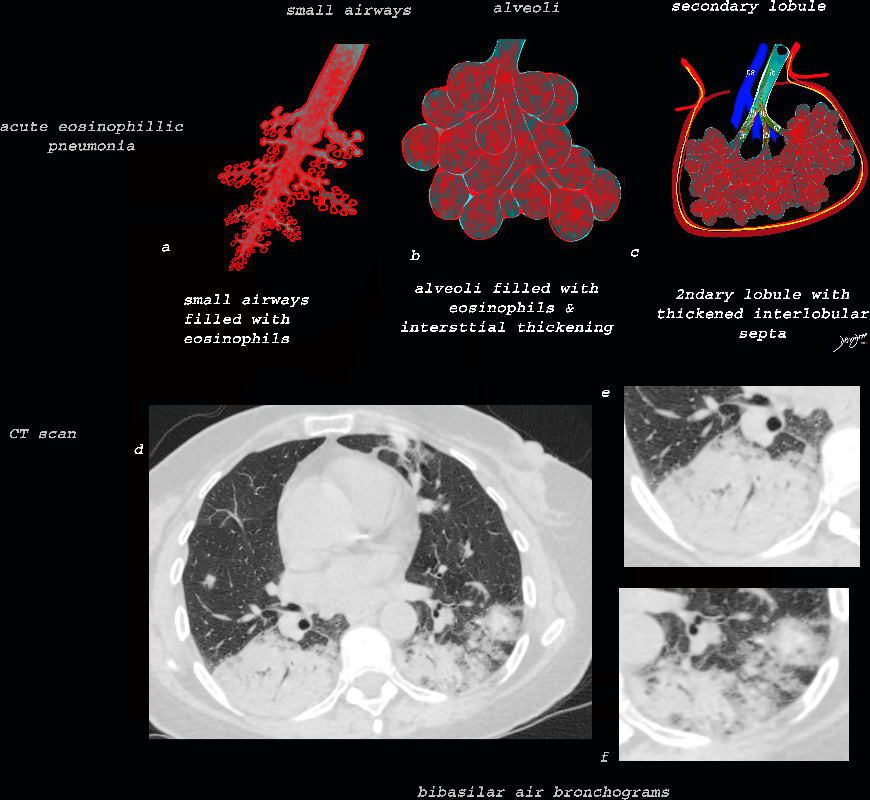
Acute Eosinophillic Pneumonia with Involvement of Small Airways, Alveoli and Interlobular Septa
Ashley Davidoff TheCommonVein.net lungs-0757b
Small Airways Infiltration with Eosinophils and Inflammatory Exudate – Centrilobular Nodules
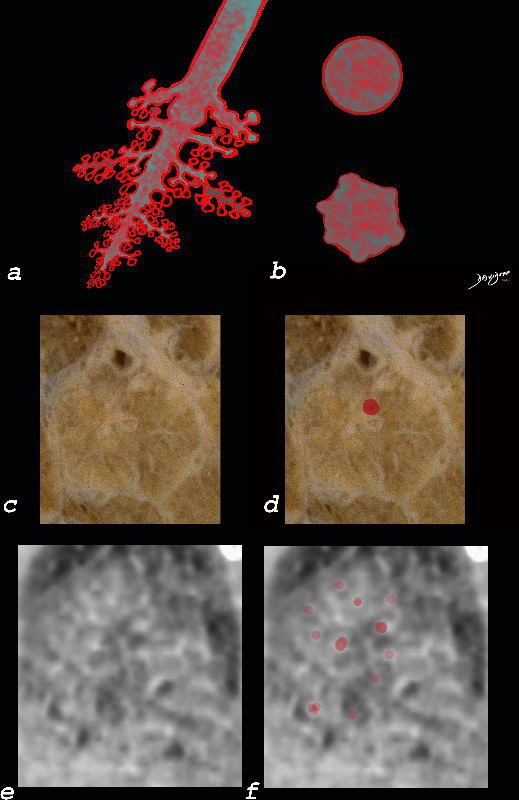
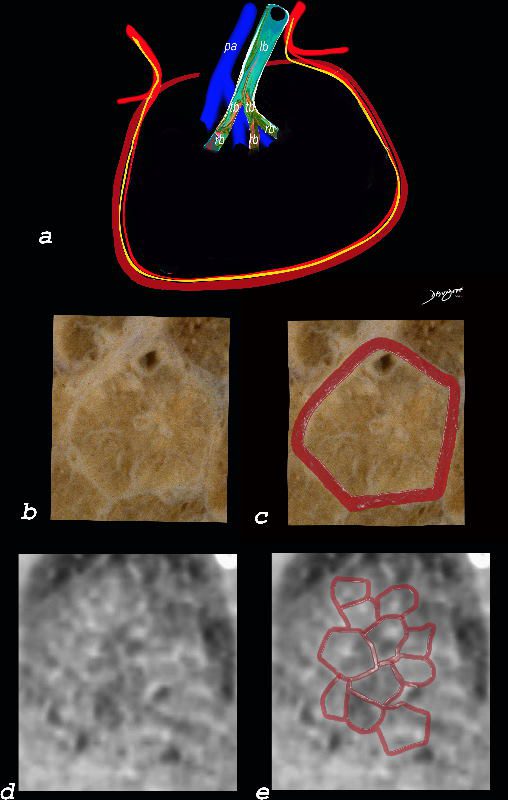
The diagram shows the small airways of the lung including the respiratory bronchiole, alveolar ducts and alveolar sacs in coronal (a) and in cross section (b) and correlated with an anatomic specimen of a secondary lobule that contains a thickened interlobular septum . The respiratory bronchiole is overlaid in maroon (d), next to the arteriole. Images e and f are magnified views of a CT of the lungs in a patient with acute eosinophillic pneumonia and the centrilobular nodules reflecting small airway disease are highlighted in f.
Ashley Davidoff MD The CommonVein.net lungs-0760b
Interlobular Septal Infiltration with Eosinophils and Inflammatory Exudate – Thickening of the Interlobular Septa – Crazy Paving Kerley B lines
The diagram shows the thickened septum surrounding the secondary lobule due to an inflammatory process, cellular infiltrate and congestion of the venules and lymphatics in the septum (a) . An anatomic specimen of a secondary lobule from a patient with thickened interlobular septa is shown in c and overlaid in d. CT of the lungs in a patient with acute eosinophillic pneumonia shows thickened interlobular septa and centrilobular nodules and the thickened septa are overlaid in red (e).
Ashley Davidoff MD The CommonVein.net lungs-0761
Alveolar and Interalveolar Interstitial Infiltration with Eosinophils and Inflammatory Exudate – Ground Glass Changes
The ground glass changes are a combination of the cellular and exudative inflammatory response in the small airways, alveoli, interalveolar septa and interstitium, and thickened alveolar septum
The ground glass changes are a combination of the cellular and exudative inflammatory response in the small airways, alveoli, interalveolar septa and interstitium, and thickened alveolar septum
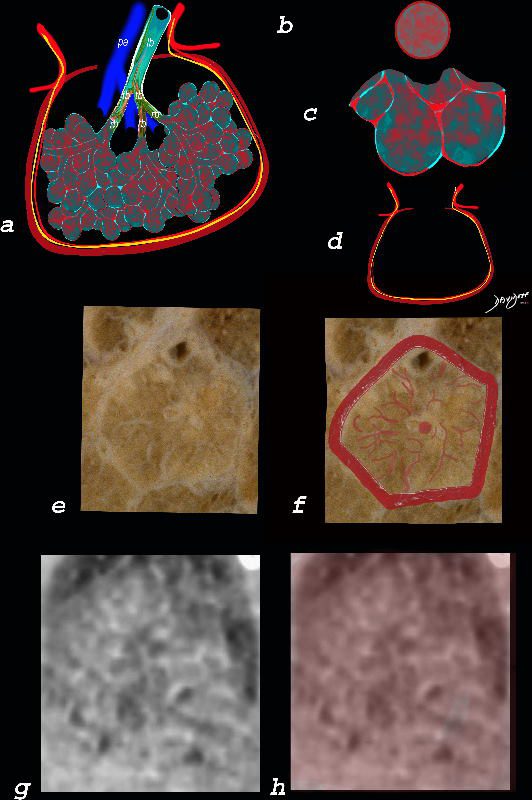
The diagram shows the abnormal secondary lobule (a) The involved components include the small airways(b) alveoli and interalveolar interstitium (c) and the thickened interlobular septum (d) surrounding the secondary lobule due to an inflammatory process, cellular infiltrate and congestion of the venules and lymphatics in the septum. An anatomic specimen of a secondary lobule from a patient with thickened interlobular septa and interstitial thickening is shown in image e, and is overlaid in red (f) . A magnified view of an axial CT of the lungs in a patient with acute eosinophillic pneumonia shows thickened interlobular septa and centrilobular nodules (g) The inflammatory changes in the aforementioned structures result in an overall increase in density of the lung manifesting as ground glass changes (g) and overlaid in red (h)
Ashley Davidoff MD The CommonVein.net lungs-0762
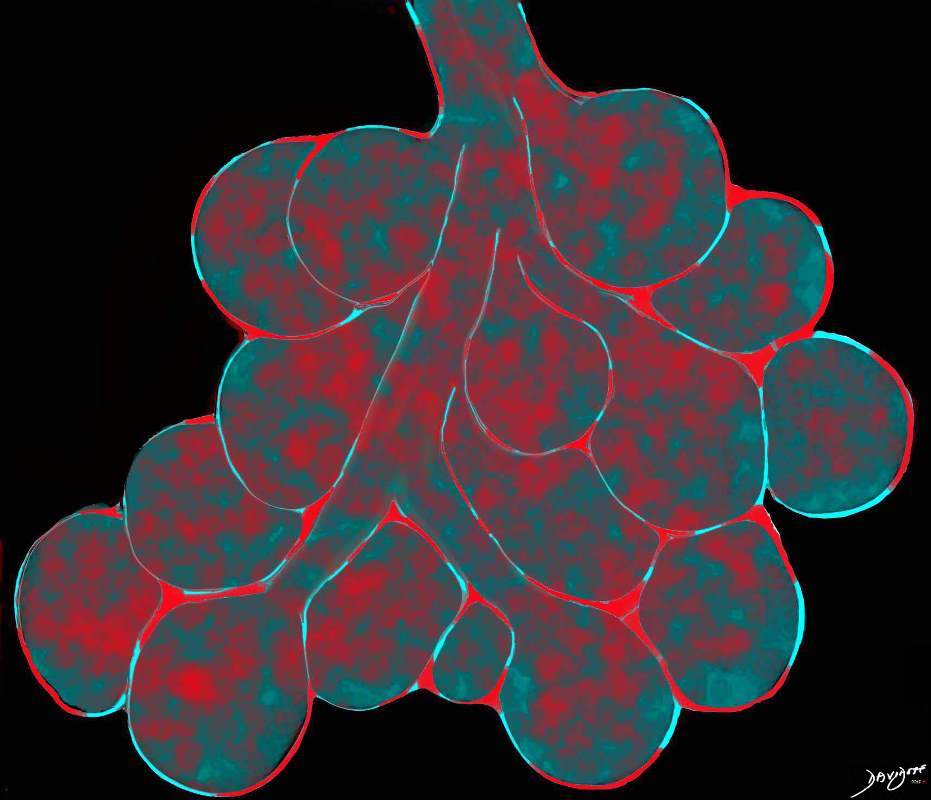
Ashley Davidoff TheCommonVein.net
lungs-0756b01
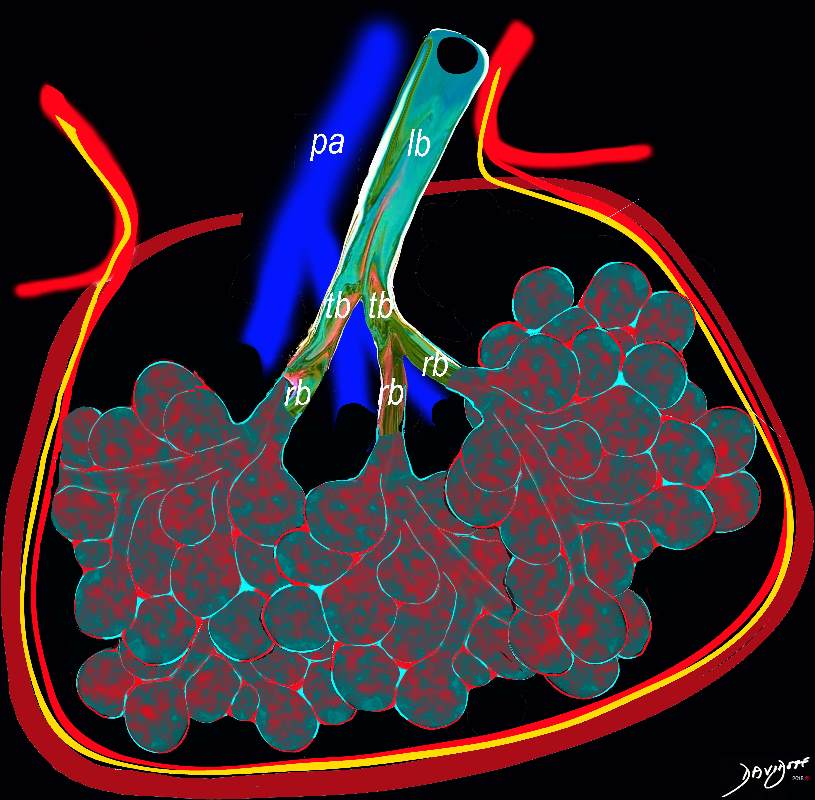
This diagram reveals the important structural changes in the secondary lobule that includes filling of the alveoli with eosinophils and proteinaceous and fibrinous exudate as well as infiltration into the alveolar septa and interstitium (red walls of alveoli) . An important component of the disease is the thickening of the interlobular septa (maroon) which results in Kerley B lines and an interstitial pattern on the CXR and CT that is reminiscent of cardiogenic interstitial edema.
Ashley Davidoff TheCommonVein.net lungs-0758
Matsuno O et al Respiratory Medicine Volume 101, Issue 7, July 2007, Pages 1609-1612
Advancing Acute Eosinophilic Pneumonia which may go onto Diffuse Alveolar Damage and ARDS
As the disease advances the small airways, and alveoli, get progressively filled with eosinophils, inflammatory cells and fluids resulting in consolidation. This image reveals progressive filling of the small airways, (a) alveoli, (b) and secondary lobules (c) with eosinophils and products of inflammation resulting in multi-segmental consolidations (d), in the lung bases, with air bronchograms at the right base (e), and less dense consolidation at the left base (f)
Ashley Davidoff MD The CommonVein.net lungs-0763
The ground glass changes are a combination of the cellular and exudative inflammatory response in the small airways, alveoli, interalveolar septa and interstitium, and thickened alveolar septum
The diagram shows the abnormal secondary lobule (a) The involved components include the small airways(b) alveoli and interalveolar interstitium (c) and the thickened interlobular septum (d) surrounding the secondary lobule due to an inflammatory process, cellular infiltrate and congestion of the venules and lymphatics in the septum. An anatomic specimen of a secondary lobule from a patient with thickened interlobular septa and interstitial thickening is shown in image e, and is overlaid in red (f) . A magnified view of an axial CT of the lungs in a patient with acute eosinophillic pneumonia shows thickened interlobular septa and centrilobular nodules (g) The inflammatory changes in the aforementioned structures result in an overall increase in density of the lung manifesting as ground glass changes (g) and overlaid in red (h)
Ashley Davidoff MD The CommonVein.net lungs-0762
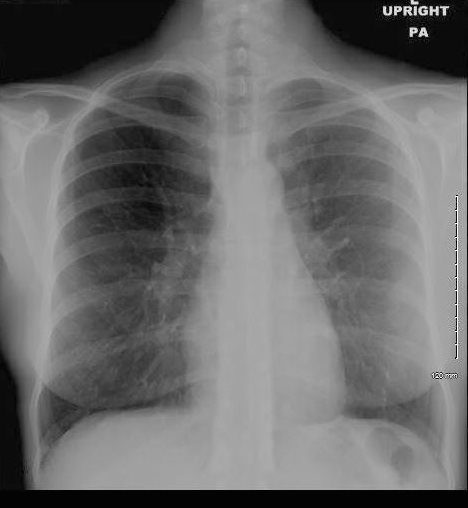
36 year old Patient in Second Phase of Disease showing Multicentric Infiltrates
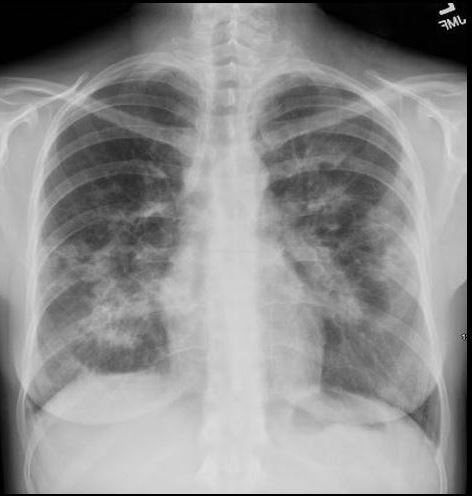
CXR shows bibasilar patchy infitrates
She was treated with antibiotics
Ashley Davidoff MD TheCommonVein.net dx acute eosinophillic pneumonia
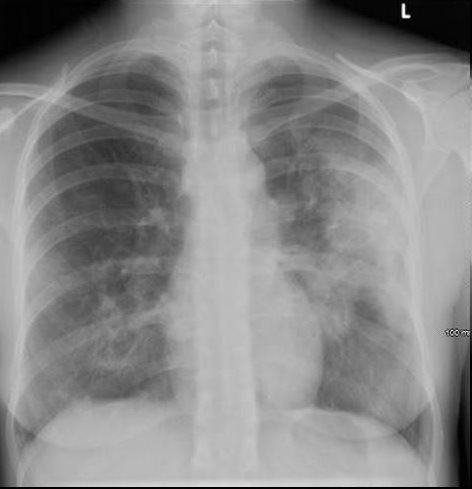
Ashley Davidoff MD TheCommonVein.net dx acute eosinophillic pneumonia
Ashley Davidoff MD
TheCommonVein.net
dx eosinophillic pneumonia
Ashley Davidoff MD
TheCommonVein.net
dx eosinophillic pneumonia
Ashley Davidoff MD TheCommonVein.net dx eosinophillic pneumonia
- Cause
- unknown
- ?acute hypersensitivity reaction to an unidentified inhaled antigen in an otherwise healthy individual
- Of all inhalational causes of AEP, tobacco smoking has been the most frequently implicated trigger
- smoke
- cigarettes
- marijuana
- electronic cigarettes
- water pipe cigarettes
- cocaine and other drugs
- immune therapy
- smoke
- unknown
- result
-
- degranulation of activated eosinophils
- granules release
- cationic proteins into the
- extracellular space,
- direct toxicity to the
- heart,
- brain, and
- bronchial epithelium
- Also release
- proinflammatory cytokines
- Structural
- extensive disease
- eosinophils
- marked numbers of interstitial eosinophils
- lesser numbers of alveolar eosinophils
- intraalveolar fibrinous exudate (100 percent of cases),
- perivascular and intramural inflammation without necrosis
- DAD
- hyaline membranes
- edema
- fibrosis
-
- Clinical
- acute/subacute illness of
- less than four weeks duration
- cough
- dyspnea
- fever (often high)
- may require ventilation
- acute/subacute illness of
- Lab
- leukocytosis
- eosinophils not initially but
- becomes markedly elevated subsequently
- may be absent or delayed, especially in smoking-related AEP.
- IgE elevated
- Some ANCA positive
- some positive sputum eosinophilia
- At the onset
- Radiology
- diffuse disease
- unlike chronic eosinophilic pneumonia, in which the opacities are typically localized to the lung periphery.
- CXR
- early chest radiograph
- AEP mimics
- hydrostatic pulmonary edema,
- subtle reticular or ground glass opacities,
- Kerley B lines
- AEP mimics
- evolving
- ground glass
- bilateral diffuse diffuse parenchymal opacities
- early chest radiograph
- CT
- bilateral, random, and patchy ground-glass or reticular opacities
- show random cephalocaudal distribution
- centrilobular nodules and air-space consolidation are seen in approximately 50 and 40 percent, respectively
- bilateral ground-glass areas: common
- interlobular septal thickening: common
- pleural effusions: can be present in ~80% (range 60-100%) of cases
- thickening of bronchovascular bundles: present in around two-thirds of cases
- air-space consolidation: present in around half of the cases
- ill-defined centrilobular nodules: present in around one-third of cases
- Bronchoscopy BAL
- Crucial for the diagnosis
- pulmonary eosinophilia in the BAL fluid
- diffuse disease
Links and References
Cottin V et al Eosinophilic Pneumonia Orphan Lung Diseases. 2014 Dec 11 : 227–251.
Daimon T et al Acute eosinophilic pneumonia: Thin-section CT findings in 29 patients Volume 65, Issue 3, March 2008, Pages 462-467
De Giacomi F et al Acute Eosinophilic Pneumonia. Causes, Diagnosis, and Management American Journal of Respiratory and Critical Care Medicine Volume 197, Issue 6
Johkoh T, Müller NL, Akira M, Ichikado K, Suga M, Ando M, et al. Eosinophilic lung diseases: diagnostic accuracy of thin-section CT in 111 patients. Radiology 2000;216:773–780
TCV
-
- Eosinophilic lung disease
-
- Systemic
- Eosinophilic granulomatosis with polyangiitis (EGPA) aka Churg Strauss Disease
- Hyper-eosinophilic syndrome
- Lung limited diseases
- Systemic
-
- Eosinophilic lung disease