Introduction
The devil is in the detail. The following module presents the anatomic detail in greater depth so that the finer points of diagnosis, particularly related to imaging and imaging techniques can be elaborated. What is the indication for high resolution CT for example? How do we time the bolus to optimize pulmonary arterial imaging? What anatomical and physiological facts do we use? Why do we change the position of the patient in the evaluation of interstitial lung disease? What is an air bronchogram, and what is ground glass opacity? What is the future for the plain film of the chest?
Components of the Lung
The detailed anatomy of the right and left lung can be found in
Part 1-Applied Anatomy of the Lung: Basic Principles.
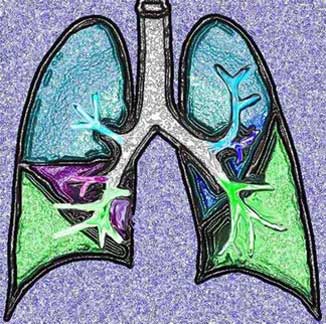
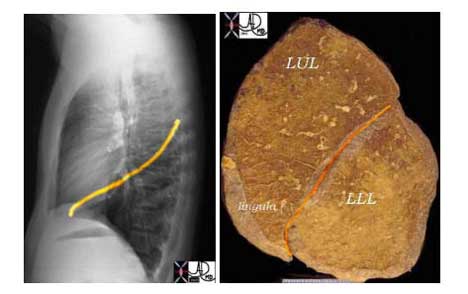
For continuity of thought we will pictorially illustrate the parts of the lung.The lung is divided into a right and left side with the right lung being composed of an upper, middle, and lower lobe, and the left lung being composed of an upper lobe (with the lingula as part of the upper lobe) and the lower lobe.

Courtesy of: Ashley Davidoff, M.D.
32558b02
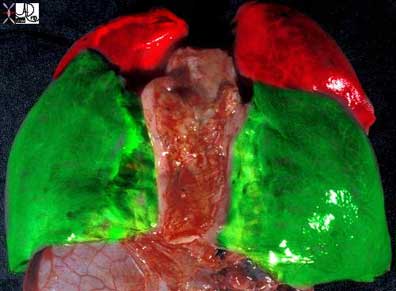
The same specimen as seen above is viewed from its posterior aspect showing the upper lobes in red and the lower lobes in green. Note that the lower lobes have a majority of their parenchyma posteriorly while the upper lobes are dominantly positioned anteriorly.
Courtesy of: Ashley Davidoff, M.D.
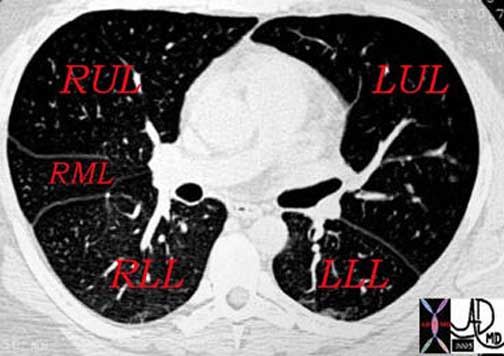
32557b01The lobes are subdivided into segments that are determined by the branching of the main bronchi. The right lung usually has the segments subtended by ten segmental bronchi. The right upper lobe (RUL) has three segments called the apical, posterior, and anterior segments. The right middle lobe (RML) has two segments named the lateral and the medial segments. The right lower lobe (RLL) has five segments also named according to position: superior, anterior basal, lateral basal, posterior basal, and medial basal segments. The superior segmental bronchus is the first branch of the RLL system and it is directed posteriorly. The superior segment is vulnerable in the supine patient who aspirates because of its posterior position.
The left lung is smaller than the right and has eight segments compared to the ten segments on the right. The upper lobe of the left lung has superior and lingula divisions. Both of these divisions have two segments each. The segments of the superior division are the apical-posterior and the anterior segments. The lingula is divided into superior and inferior segments. The division of the lower lobe closely resembles that of the right except that there is consolidation of two of the left lower lobe segments. Thus, while the RLL has five segments, the LLL has only four.
The segments are divided into the secondary lobules. The lobules are made up of the small airways including the terminal bronchioles, respiratory bronchioles, alveolar ducts, alveolar sacs and the alveoli themselves.
Ashley Davidoff MD
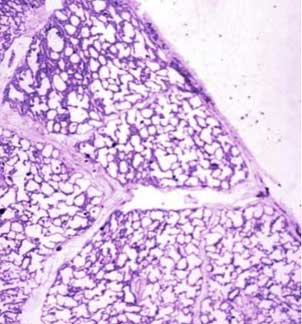
This drawing shows about 3-4 respiratory bronchioles that serve to make a secondary lobule. Alveolar sacs and individual alveoli are also seen. The yellow border represents the visceral pleura on the surface.
Courtesy of: Ashley Davidoff, M.D.
This drawing shows about 3-4 respiratory bronchioles that serve to make a secondary lobule. Alveolar sacs and individual alveoli are also seen. The yellow border represents the visceral pleura on the surface.
Courtesy of: Ashley Davidoff, M.D.
Size and Surface Anatomy
The lungs are one of the more voluminous organs in the body but in terms of weight are relatively light for their size, since they are filled mostly with air. Additionally, their size can change dramatically since the difference in volume between a deep inspiration and deep expiration can be as much as 3.5 liters.
Within the thorax the lungs extend superiorly passed the clavicle into the base of the neck, with a craniocaudal length in inspiration of approximately 24 cm. Posteriorly, on a chest X-ray they reach to the 10th rib. Cranially they extend to the first thoracic vertebrae and inferiorly to the dome of the diaphragm and costophrenic sulci more inferiorly.
In patients with COPD (chronic obstructive pulmonary disease) or an acute asthmatic attack, the lung volumes are expanded and the lungs will be seen beyond the 6th rib anteriorly and the 10th rib posteriorly.
Weight and Volumes
In the living and breathing adult, the lungs weigh approximately 900 to 1200 grams, of which nearly 40% to 50% is blood. The weight will depend on how much blood is running through the lungs. At rest it is 5L/min, but during exercise, the blood flowing through the lungs can be up to 25L/min and at any one time during exercise, the lungs will be that much heavier. At rest the lungs together weigh about the same as the liver, which is 1/3 the volume of the lungs.
The left lung is smaller since the heart, which is dominantly a left sided structure, occupies part of the left chest.
The total volume of the lungs is about 6300ml in adult men and 4200ml in adult women. At end-expiration, the volume of air within the lungs is about 2.5 L, whereas at maximal inspiration it may be 6 L. Taking a rate of 10-15 breaths per minute (about 17,000 breaths per 24 hours) results in approximately 10,000 liters of air inspired per day. Of course an equal amount has to be expired per day as well, and so there is the back and forth movement of about 20,000 liters per day.
Alveoli make up approximately 50% of entire lung volume. The outer 1/3 of the lung contains 50% of the alveoli. Thus alveoli are the major component of the lung and they are situated dominantly in the periphery while the tubular transport systems are located centrally by the hilum. The lung and the kidney have structural similarities in that the outer third of the kidney called the cortex contains most of the key functioning and filtering aspects of the kidney, whereas the inner and more central medulla houses the tubular system. The similarity is such that some refer to the lung as having an outer cortex and an inner medulla.
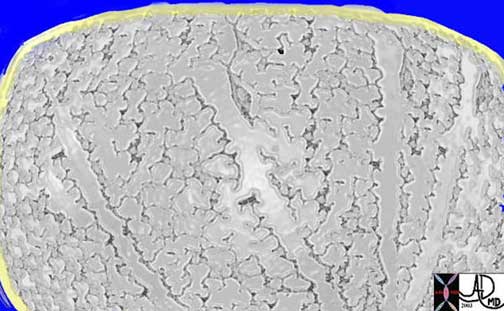
A coronally reconstructed CT image showing the effect of the normal left sided position of the heart on the volume of left lung. Note how much smaller the left lung is compared with the right.
Courtesy of: Ashley Davidoff, M.D.
Surface Area
There are about 20,000 acini and 300 million alveoli in the normal lung. During inspiration the radius of the alveolus doubles from about 0.05 mm to 0.1 mm. The total surface area of the lung is about 80 square meters, about the size of a tennis court. The extent and volume of air that is moved across the alveoli is simply stunning.As we have already seen about 5-10 liters of air is moved through the tracheobronchial tree per minute at rest. During exercise up to 100 liters of air can be moved through the airways per minute and about 3 liters of oxygen and 3 liters of carbon dioxide moved across the membrane. There are many factors that allow for the exchange to take place but the lung does its part by providing a large patent surface area by virtue of its alveolar structure.
The lungs create an increased space by bubbling, or ballooning out in the form of the alveoli. The gastrointestinal tract and kidney have more time to do their work. The lungs need all the time they can get since most of the exchange is done during inspiration and even more effectively between inspiration and expiration. The air that is inspired must present itself to the functional surface at large almost immediately, and the exchange has to take place before expiration starts when the air is whisked away to prepare for the next load.
Surface Area: Applied Anatomy
In the clinical realm, there are certain diseases that make the lungs abnormally large and those that reduce the size of the lungs or segments of the lungs.
The lungs will usually take up the available space in the chest so that if one part of the lung is collapsed or surgically removed then the remaining lung will take up the space. Similarly, if one lung is blocked or becomes smaller, then the other becomes hyperinflated resulting in shape and size changes to accommodate the deficiency.
In the COPD group, including emphysema, there is air trapping so that what goes in does not come out because of the structural changes in the lung, resulting in hyperinflation and large lungs. In an acute asthmatic attack there is air trapping making the lungs larger in volume than normal. The diaphragm gets pushed down and becomes flattened. Atelectasis is also part of the disease because thickened mucus plugs the smaller airways, and therefore air cannot get in and subsegmental collapse occurs. Less commonly the mucus may block the airways at segmental and lobar level with significant consequences to an already compromised system. The diaphragm on the same side of the collapse becomes elevated to the degree that depends on the size of the collapse. The larger the collapse the greater the volume loss in the lungs, the greater the space that needs to be filled in, and hence the greater the elevation of the diaphragm. The concept of filling in space is also witnessed when a patient has had resection of part of the lung. The space usually is filled in by the remaining segments of the ipsilateral lung, but if there is insufficient ipsilateral lung to do the job, (fill in the space) it may be filled in by contralateral lung.

Courtesy of: Ashley Davidoff, M.D.
Courtesy of: Ashley Davidoff, M.D.
Shape
The lungs are funnel, conical, or even pyramidal in shape, with a narrow apex and a broad, concave base.
The apex, also called the cupola is dome shaped, fitting snuggly into the space created by the soft tissue confluence of the mediastinal parietal pleura with the costal parietal pleura and the bony frame formed by the clavicle and first rib. It is positioned 2-3cm superior to the medial third of the clavicle where it projects through the superior thoracic inlet.
Courtesy of: Ashley Davidoff, M.D.
Lung parenchyma is relatively exposed at the apex making it vulnerable to penetrating injuries of the neck.The base of the lung is concave so that there is a snug fit with the hemidiaphragms bilaterally. An intimate and parallel contact is maintained during all phases of respiration. This contact is necessary since the function of these two structures is intimately linked. The shape of the base will of course change with respiration and, like two dancers doing the tango, the diaphragm and lung move gracefully, intimately, and sometimes dramatically together with each phase of respiration – even with the sharp turn of events of a hiccup or a cough (or the tango equivalents – the gancho or the giro!).
Each lobe of the lungs has a characteristic shape, and may look very different in the A-P projection and lateral projection. The right upper lobe (RUL) is roughly triangular in shape but with blunted or rounded angles. Its A-P shape is very similar to its lateral shape. The right middle lobe (RML) and the right lower lobe (RLL) are both rectangular in the A-P projection but triangular in the lateral view, (apex at hilum and base anteriorly), though the RML is comparatively smaller.
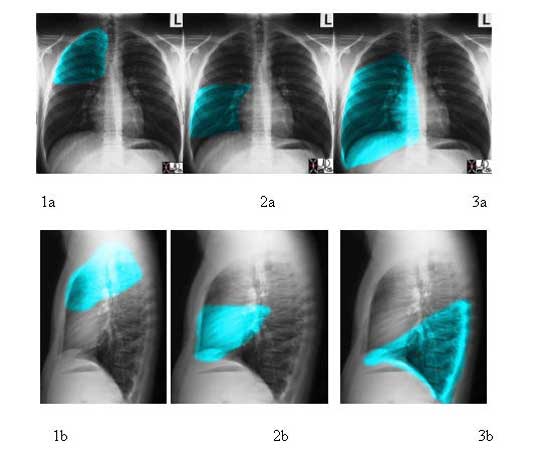
Courtesy of: Ashley Davidoff, M.D.
The left upper lobe (LUL) from the A-P projection resembles a rectangle with the upper edge being rounded, while from the lateral view it is shaped like half an oval, the bottom part being pointed. The left lower lobe (LLL), from the A-P projection, is almost a perfect rectangle, and from the lateral view is almost a perfect triangle.
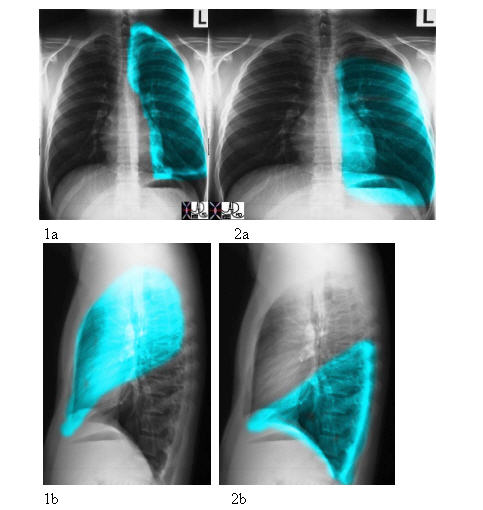
Courtesy of:Ashley Davidoff, M.D.
Shape: Applied Anatomy
In a hyperinflated state such as exists in emphysema, the lungs are full, bulging with air, and less pliant, causing the diaphragm to flatten. Clinically, the patient is described as having a barrel shaped chest, a pigeon shaped chest, or in Latin, pectus carinatum. The chest X-ray (CXR) in this circumstance will show flattened hemidiaphragms, reflecting the almost fixed shape of the lungs, and an increase in the air space behind the sternum, (the retrosternal air space) which causes the barrel shape.
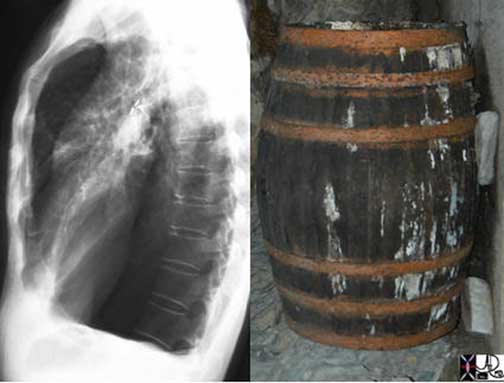
Courtesy of: Ashley Davidoff, M.D.
Courtesy of: Ashley Davidoff, M.D.
Position
The lungs are located within the thorax, within the thoracic cavity and within the pleura. They occupy about 3/4 of the thoracic cavity, with the rest occupied by the heart and the mediastinum. The right lung occupies almost the entire right hemi thorax and the left is displaced by the heart and occupies a lesser amount of space.
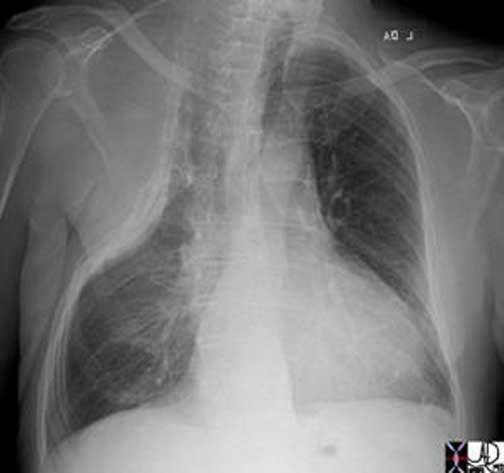
The lungs are divided into upper and lower lobes being somewhat of a conceptual misnomer, since the lower lobes creep up posteriorly to almost the apex of the lungs and the upper lobes (together with the lingula and middle lobe) reach more inferiorly in the anterior location. The lobes may have just as well been called the posterior lobes and the anterior lobes but because the upper lobes reach the most superior position in the chest and the lower lobes the most inferior and diaphragmatic parts, they were reasonably labeled upper and lower.
Courtesy of: Ashley Davidoff, M.D.
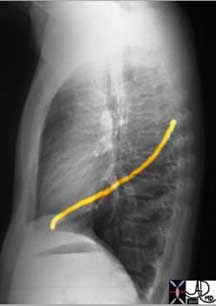
Courtesy of: Ashley Davidoff, M.D.
The segments are named according to their position, and include the apical anterior and posterior segments in the upper lobes, the lateral and medial components of the RML, the superior and inferior of the lingula, and the superior (apical) lateral medial anterior and posterior basal segments. Note that all the names relate to the position of the segments. There is a move to give these segment numbers – but it seems far easier to understand the spatial positioning by naming the segments according to their position.
The middle lobe has a very intimate relation to the right heart border (RA), while the lingula has a similar relationship with the left ventricle. This has relevance in the evaluation of infiltrates on the CXR since disease in the RML will cause silhouetting of the right heart border (RA), while an infiltrate in the lingula will silhouette the left heart border. On the lateral examination since both of these are anterior segments they will be seen as anterior infiltrates. Hence the position of infiltrates as seen on the CXR can be accurately localized to segments by looking at the relationship to the heart in the P-A and whether the infiltrate is anterior or posterior on the lateral.
Courtesy Ashley Davidoff, M.D.
Position: Applied Anatomy
Situs inversus (reversal of position or location) has a wide variation of clinical presentations. Some patients with situs inversus may live a completely normal life without ever being aware of the condition. At the other extreme, the aberrant morphology, particularly related to the heart is life threatening.
Courtesy of: Ashley Davidoff, M.D.
Courtesy of: Ashley Davidoff, M.D.
Anatomy of the Structures Surrounding the Lungs
The lungs are completely surrounded by the pleura, a variety of muscles and the bony thoracic cage. The heart and other structures of the mediastinum form the medial relations of the lungs.
Within the mediastinum lies the heart and pericardium, the trachea and main stem bronchi, esophagus, thoracic duct, lymph nodes, and both phrenic and vagus nerves. The mediastinum is situated anterior to the sternum and ventral to the spinal column, nestled between the pleural sacs of the right and left lungs. Superiorly it reaches the level of the fourth thoracic vertebrae and rests upon the diaphragm inferiorly. Below the diaphragm lie the liver, gall bladder, and spleen.
Surrounding the lungs are the thoracic muscles and the diaphragm itself. These are the muscles related to respiration. Their operation alters the pressure within the thorax. When the thorax expands, the pressure decreases allowing air from the trachea into the lungs. When it contracts, the lungs compress and air leaves the lungs. When the diaphragm contracts, it flattens and sinks as much as 7-10 cm below its normal level at rest. As the muscles relax the thorax returns to normal pressure and air from the lungs is released. This is known as abdominal respiration and accounts for approximately 70% of respiration in a resting body.
Courtesy of: Indiana University and Philips Medical Systems
Character of the Lungs
Leonardo da Vinci in the late 15th century accurately depicted the spongy and elastic nature of the lungs by the following observation.”The substance of the lung is dilatable and extensible like the tinder made from a fungus. But it is spongy and if you press it, it yields to the force which compresses it, and if the force is removed, it increases again to its original size.”
The lungs are filled with air, and they look and feel like a soft sponge. Their infrastructure of thin septa and air filled cavities is very reminiscent of the architecture of a sponge. The lung “crepitates”, or has a characteristic “crackling” feeling when handled. If it is inflated with air ex vivo, the healthy lung will float. The value of the relative amounts of air, and hence floatability, is well known to swimmers and snorkelers who can control their buoyancy by titrating the amount of air in their lungs against where they want to passively float in the water.
The chest wall and lungs are also characterized by an elastic nature allowing the apparatus to recoil after inspiration. The act of expiration is thus a passive process requiring no energy as the elastic recoil brings the lung back to unexpanded state.
Courtesy of: Ashley Davidoff, M.D.
Color
The color of the healthy lungs is affected by age and geographic location. In the young, they are pink and healthy appearing. In an industrial society the lungs are directly exposed to a smoky environment, and carbon particles are retained in the lungs and lymph nodes giving the lungs a dark slate gray color with a mottled appearance. With advancing age, the slate gray mottling changes to a black. The carbon-type granules are mostly deposited in the tissue near the surface of the lung.

Courtesy of: Ashley Davidoff, M.D.
Courtesy of: Ashley Davidoff, M.D.
32291
The Mediastinum
The mediastinum is densely populated with important structures including the aorta, esophagus, trachea and heart. Often the first signs of disease are recognized on the chest X-ray as a result of subtle changes in the shape of the mediastinum. It is therefore imperative that radiologists are extremely familiar with the normal shape of the mediastinum. Review of the shape may start with the left side at the apex – Think of yourself skiing down a mogul trail and consider the bumpy ride that may challenge you. There is a left sided slope and the right sided slope – both are black diamonds. Get your skis and helmets on– these are tough slopes.
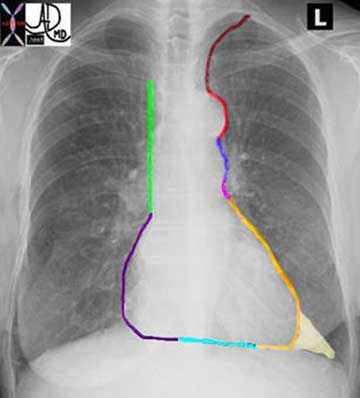
The second slope on the right starts near the apex of the right lung and is marked with a bright green sign called “vein cave”. As you step off the lift – there is a ninety degree drop, and if you look to your left you will see the red cells in the superior vena cava traveling much slower than you. After the “vein cave” route, the gentle curve around the right atrium (purple) takes over and you are brought to an almost negligible slope of the right ventricle (teal). The right and left slope meet at the bottom by the ski house.
Courtesy of: Ashley Davidoff, M.D.
The Mediastinum: Applied Anatomy
The following image represents one of the many shape changes that occurs in the mediastinum that will tip the radiologist off to a disease process in the chest.
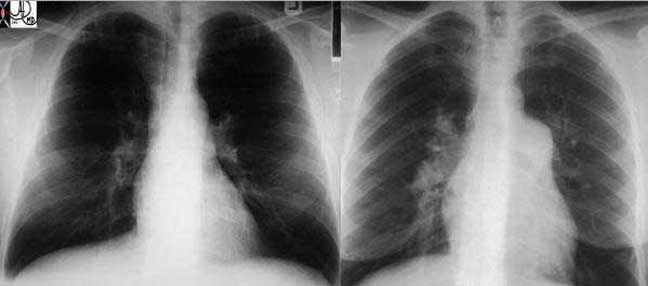
Courtesy of: Ashley Davidoff, M.D.
Courtesy of: Ashley Davidoff, M.D.
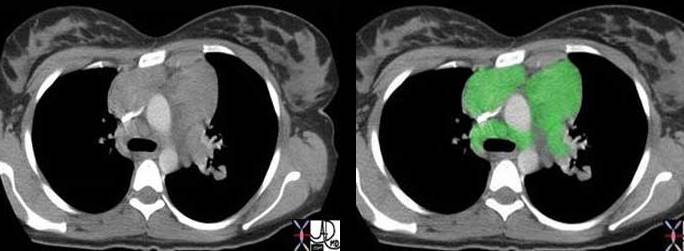
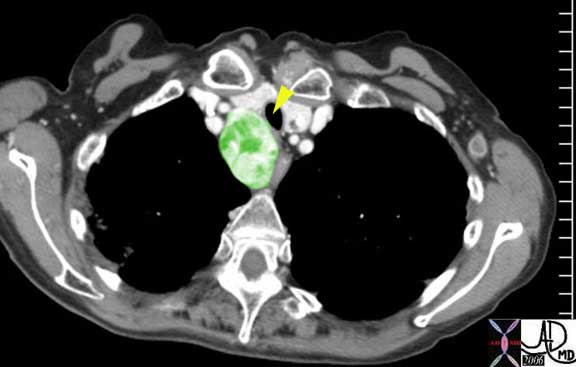
Right image: The enlarged lymph nodes are outlined in green.
Courtesy of: Ashley Davidoff, M.D.
The Trachea
The trachea is a tubular portion of the respiratory system that connects the larynx to the bronchi. It is relatively thin walled, about 6 inches long in the adult, consists of a series of c-shaped cartilaginous rings anteriorly and a membrane posteriorly. At the level of the carina it divides into left and right bronchi.
The trachea, although it is a tube, is made up of 15 – 20 C-shaped cartilaginous rings with a posterior membranous surface, giving it a horseshoe shape in cross section. Its A-P dimension is thus longer than its transverse dimensions.
The shape of the tracheobronchial tree at large is very similar to a branching tree. If you took an image of the tracheobronchial tree and turned it upside down you would see the following. It has an irregular dichotomous branching pattern meaning it divides into paired branches of unequal length and diameter.
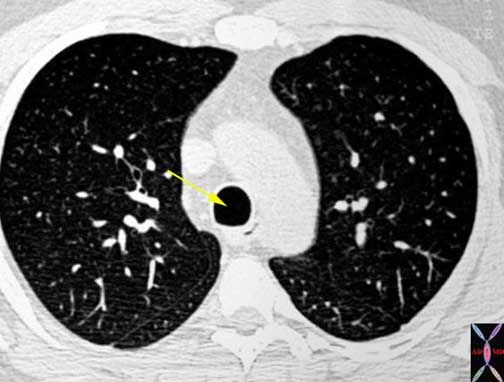
Courtesy of: Ashley Davidoff, M.D.
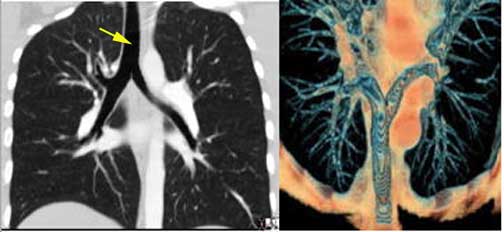
Courtesy of: Ashley Davidoff, M.D.
The Trachea: Applied Anatomy
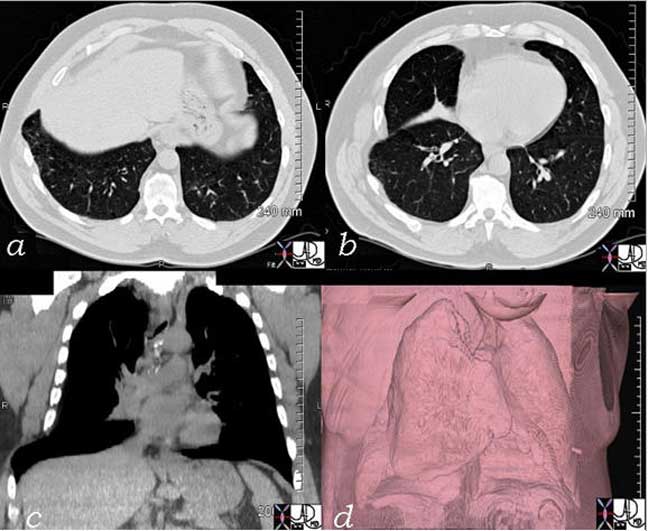
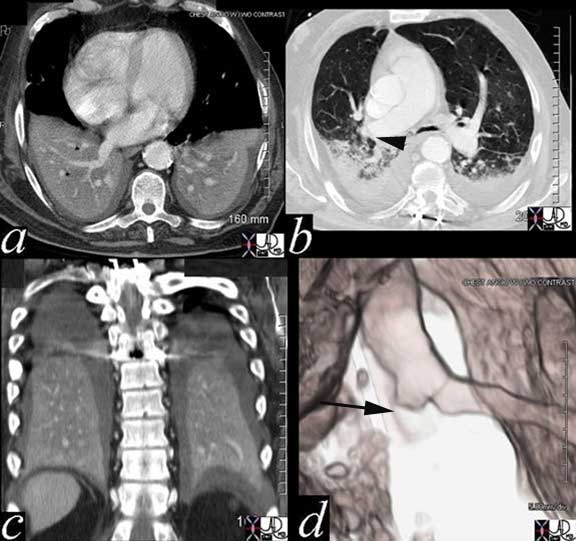
Tracheomalacia is softening of the tracheal cartilages which results in relative collapse of the trachea during inspiration and thus affecting airflow. The cause can be due to congenital weakening of the cartilage, due to extrinsic disease such as vascular rings and slings, or acquired from prolonged intubation or chronic infection. (eMedicine).
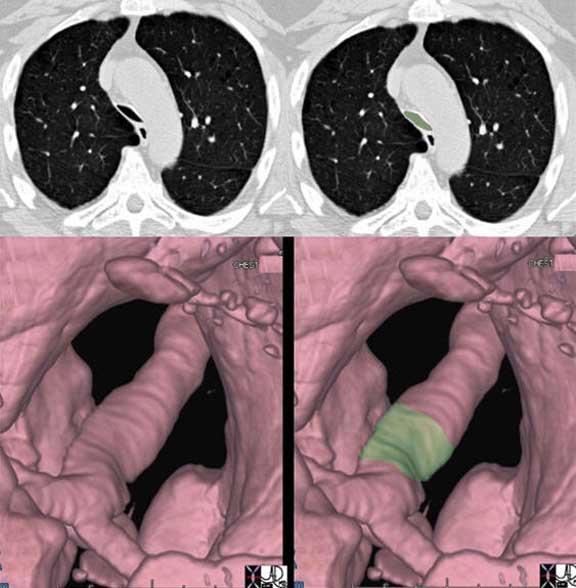
The trachea narrows significantly in its A-P dimension above the carina as seen in the cross sectional CT (a, b) with the narrowing noted in green overlay, and the change in diameter is better appreciated in the surface rendering of the trachea in (c) and (d).
Courtesy of: Ashley Davidoff, M.D.
The Trachea: Position of the Trachea
The trachea is central in its position, and lies anterior to the esophagus. It originates below the pharynx and ends at the carina where it bifurcates into left and right main stem bronchi.
Courtesy of: Ashley Davidoff, M.D.
Bronchi and Bronchioles
The mainstem bronchi taken together have 40% more cross sectional area than the trachea. This progressive increase in cross sectional area continues as we progress downstream resulting in an overall reduction in resistance, evolution of laminar flow, and facilitation of airflow. This structural feature allows air from a single breath to reach all the alveoli almost simultaneously.There is considerable discrepancy in diameter and length between the right and left mainstem bronchi. The right mainstem bronchus is short and plump while the left mainstem bronchus is the long and thin.
The right mainstem bronchus measures about 1.4 cms in diameter while the left mainstem bronchus is about 1.3cm both being slightly smaller in the female (Hampton , et al).
The RUL bronchus is about 1cm in diameter, with the apical segment being 4-7mm, and the RML bronchus is about 8mms.
The LUL bronchus resembles its uncle, the right main stem bronchus, since it is also relatively short and fat possibly because it is responsible to give rise to the lingula as well as the left apical segments. It measures 11mm in diameter (but only 9mm in length). The ascending upper division bronchus is approximately 7mm in diameter.
Bronchi and Bronchioles: Applied Anatomy
The failure of air delivery is one of the major causes of fatality during an anaphylactic reaction or a severe asthma attack. Return of airway patency is the first consideration in resuscitation. In fact, in any resuscitation, the “A” of the “ABC” of resuscitation is to ensure patency of the airway first. Maintenance of airway diameter is provided by cartilage in the upper components of the airways. In the trachea a C-shaped cartilaginous ring surrounds the anterior and lateral walls while the posterior wall consists of a membrane allowing for some pliability during the phases of respiration. The trachea lengthens and dilates during inspiration while it shortens and narrows during expiration.Some disease states compromise the patency of the airways. Tracheomalacia is a softening of the cartilage of the trachea and during inspiration the trachea will be unable to maintain its diameter. This will result in a relative collapse during inspiration and hence diminished air flow.
More downstream, support of airway patency transforms from cartilagenous support to muscular support. Asthma, inflammation, or infection can affect the diameter by inducing muscle spasm while the presence of edema of the wall would also result in narrowing of the lumen. In most of these diseases the airways of both lungs are diffusely involved and respiratory decompensation can easily result due to the extent of involvement. Collapse of a lobe, segment, or sub segment of a lung on the other hand may not affect respiratory function at all when the remaining lung is normal or near normal. Localized collapse may occur with inhalation of a foreign body, mucus or purulent impaction, and tumor growth into the lumen. Patients who have undergone a pneumonectomy usually have normal or near normal pulmonary function, unless there is disease in the other lung.
Courtesy of: Ashley Davidoff, M.D.

Courtesy of: Ashley Davidoff, M.D.
The Tracheobronchial Tree
The tracheobronchial tree changes its morphology (and so its nature) as it progresses from a relatively rigid cartilaginous structure strengthened by C-shaped cartilages. The cartilage accompanies and supports the bronchi but disappears at the level of the bronchioles. The structure of the walls progresses to muscular, elastic, and mucus secreting bronchioles and then to delicate one cell layered airways at the alveolar level. The bronchi contain cartilage but the bronchioles do not.
The main pulmonary artery (MPA) is usually about 3-4 cm in diameter being very similar to the ascending aorta and twice the size of the trachea. The branch pulmonary arteries are each about the same size as the trachea. Although the right and left mainstem bronchi are in combination and thus the right pulmonary artery (RPA) and left pulmonary artery (LPA) are quite a bit larger than their counterparts, the right and left mainstem bronchi. However, at the segmental level there is a catch up, so that the bronchi and arteries are equal in size.
These comparative sizes are very important in the imaging world and size evaluation is very useful in the diagnosis of tracheomalacia, COPD (big trachea), tracheal narrowing, bronchiectasis, aortic aneurysm, pulmonary congestion, and pulmonary hypertension – all common medical conditions that have characteristic changes in size that are evaluated by comparing the sizes of the airways to the vessels. It should be noted that in dependant positions the artery might normally appear slightly larger than its airway counterpart. This means that if the patient is supine, blood will be more dependent posteriorly and so the posterior vessels may be slightly larger than their bronchial brother or sister.
In the normal patient the pulmonary arterial branches and the bronchi are about the same size until they reach the hallowed halls of the pulmonary lobule.
Courtesy of: Ashley Davidoff, M.D.

Courtesy of: Ashley Davidoff, M.D.
The Tracheobronchial Tree: The Tracheobronchial Tree Continued
The reason we are able to see the air filled bronchi within the air filled lung is because they have wall that is made up of the soft tissue allowing for an interface. As the bronchi get smaller their walls will get proportionately thinner until they are too thin to resolve at which time they will blend into the parenchyma. They become invisible as discrete structures on high resolution CT when they reach 2mm in size, which corresponds to the bronchioles that are about 2 cm from the lung periphery. The arterioles on the other hand maintain their soft tissue character and their interface with air of the lung is maintained allowing detection even in subpleural regions of the lung periphery.Bronchiectasis is a condition where disease within the walls of the airways results in weakening resulting in enlargement and hence ectasia. In this condition, therefore, the bronchioles will be larger than their arteriole counterpart and they will be seen within 1 cm distance of the pleura.

Courtesy of: Ashley Davidoff, M.D.
The Tracheobronchial Tree: Length
The length of the airways does have practical significance as well, contributing to velocity of flow. Knowledge of the length of structures is also important for safe execution of procedures such as endoscopy, intubation, and stent placement.In adults, the trachea ranges from 9 to 15cm in length, terminating distally at the carina which represents the origins of the left and right mainstem bronchi. The distance from mouth to carina is about 25cm.
As previously stated, the right and left bronchus show considerable difference in their morphology. The right is short and plump while the left is long and thin.
The Tracheobronchial Tree: Important Dimensions
The right lung has 10 bronchopulmonary segments while the left has 8. There are approximately 23 airway divisions from the mainstem bronchi to the level of the alveoli.
The number of branches from hilum to periphery is variable with the shortest path to a terminal bronchiole being about 7 divisions and total length of 7 to 8 cm, and the longest pathway having about 25 branch divisions with total length of more than 22 cm. In the upstream portions of the airways the tree does not divide as frequently as they do downstream. The lungs are large organs and the aim of the airways is to deliver the air to the alveoli. Distance needs to be covered from the hilum where alveoli are relatively sparse, to the periphery where the alveoli are abundant. Prior to entering the secondary lobule, the bronchovascular bundle branches at approximately 1 cm intervals. Once they enter the confines of the lobule, branching becomes fast, furious and a new branch originates every 1 – 3mm. This frequency of division in the hallowed halls of gas exchange makes intuitive sense as surface area becomes the dominant focus.
The left main stem bronchus leaves the trachea at a 135-degree angle. The right mainstem bronchus is more vertically oriented, with a 155-degree angle of origin. The carinal angle should be about 55 degrees and should not be more than 90 degrees. In patients with subcarinal disease including pathological lymph nodes (lymphoma and metastatic lung carcinoma), left atrial enlargement (mitral stenosis, regurgitation and left heart failure), and large hiatus hernia, the carinal angle may be widened. The carinal angle is therefore a very important landmark in the evaluation of the chest X-Ray.
The Tracheobronchial Tree: Position
The trachea is central in its position, and lies anterior to the esophagus. It originates below the pharynx and ends at the carina where it bifurcates into left and right main stem bronchi. The right bronchus lies more vertical in its axis than the left. When we look at a normal CXR we find that the right hilum is slightly lower than the left. Since the hilum is made up of vessels and airways and since the RPA runs below the right mainstem bronchus, the superior most structure in the right hilum is the bronchus. The left bronchus is hyparterial, meaning that it runs below the LPA. (i.e. the LPA does the high jump over it) Thus, the left hilum is higher than the right. The RPA cannot make the jump over the right mainstem bronchus, (called the eparterial bronchus) resulting in a right hilum that is slightly lower than the left.
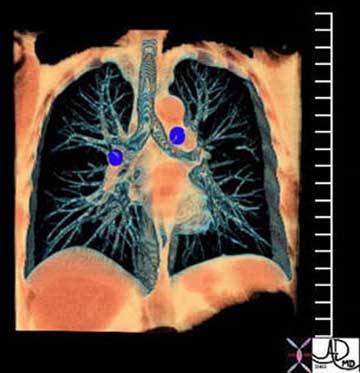
Courtesy of: Ashley Davidoff, M.D.
The Tracheobronchial Tree: Applied Anatomy
Knowledge of the relationship of the trachea and esophagus is extremely important for those who are intubating patients or placing nasogastric tubes. The trachea and esophagus lie very close to each other, and so it is not uncommon for the intubations to be misplaced.
Upper lobe disease such as tuberculosis (TB) will cause fibrosis and shrinkage of the lung structures with consequent traction effect and elevation of the hilum so that it is pulled upward.
The Pleura
The pleura consist of a double layer of glistening, semitransparent serous membranes, which surround the pleural space. The secreting surfaces face each other across the pleural space. One of these layers, the visceral pleura, is intimately attached to the lung and follows the fissures, while the second component, the parietal layer, is intimately related to the thoracic cage, mediastinum, and diaphragm. There is no communication between the right and left pleural spaces. The pleura have elastic qualities in order to accommodate the changes in size with the expansion and contraction of the chest cavity. During the respiratory cycle the two layers of pleura move in tandem gliding smoothly over each other, lubricated by a small amount of serous fluid. As the chest wall expands during inspiration, the parietal pleura moves with it, while the visceral pleura moves with the expansion of the lung, the two being held together by the negative pressure caused by capillary forces of the fluid that lies between the two smooth surfaces. Try the experiment of putting a small film of water between two clean, smooth, and flat glass surfaces. The two cannot be easily separated, unless a small amount of air can be introduced between the opposing surfaces.
The cells lining the pleura are called mesothelial cells. They are responsible for the production of pleural fluid. Although we describe two components to the pleura, it is really one structure into which the developing lung bud grows, similar to a fist pushing its way into a balloon. At the hilum of the lung, the bronchovascular and lymphatic bundles acting as the fist, push their way into the pleura and become enveloped by the pleura, with the most distal progeny of the lung bud coming into intimate contact with the visceral pleura.
Despite the apparent single embryologic origin of the visceral and parietal pleura, they are in fact functionally and structurally different. The visceral pleura, for example, have no pain fibers; whereas the parietal pleura are extremely sensitive to pain. The visceral pleura receive its nerve supply through the autonomic nervous system, whereas the parietal pleural innervation is via the somatic intercostal nerves and the phrenic nerve.
The blood supply of the visceral and parietal pleura is also different. The parietal pleura are supplied only by the systemic circulation from the intercostal arteries while the visceral pleura are supplied by the bronchial arteries and the pulmonary arteries.
The visceral pleura follow the lung surface and therefore will follow the fissures. The parietal pleura follow the chest wall and therefore will not follow the fissures of the lung. The pleural cavity is larger than the total lung volume, allowing the lung to expand during respiration. It is particularly obvious in the pleural recesses at the inferior aspect of the thoracic cavity.
Courtesy of: Ashley Davidoff, M.D.
The visceral pleura are subdivided into three distinct parts, including the mediastinal, the diaphragmatic, and the costal pleura.
There is continued production of pleural fluid by the mesothelial cells into the pleural space. There is also a continued drainage of the fluid by the subpleural lymphatic system which lies between the visceral pleura and lung coursing into the interlobular septa.
The Pleura: Applied Anatomy
On a plain film of the chest the easiest method of identifying fluid is the lateral upright examination where fluid accumulates in the posterior pleural recesses and causes “blunting” of the posterior costophrenic angles. CT is far more sensitive in the detection of the fluid and to some extent in the characterization of the fluid. When the fluid layers with a meniscus that is parallel to the floor and falls into expected dependant positions (just as water would in a glass on a flat table top) then the effusion can be expected to be free flowing. If the fluid falls in a non-dependant fashion it is considered “loculated” and it is more likely to be complex. Under these circumstances the level is tilted. Ultrasound however is better able to identify septations and other soft tissue components of the fluid collection. Thoracentesis however is still necessary to enable the chemical evaluation of the fluid.
When there is an accumulation of fluid in the pleural space (pleural effusion) or air (pneumothorax) the underlying lung gets pushed away from the chest wall and the harmonious interaction of chest wall pleura and lung is lost. As the accumulation gets larger, the underlying lung collapses without the support mechanisms, and functional lung tissue is lost, with ensuing respiratory difficulty. The lungs are relatively forgiving in that they have enough reserve to accommodate effusions (even large) and moderate degrees of atelectasis, particularly when this occurs gradually, enabling compensatory mechanisms to evolve.
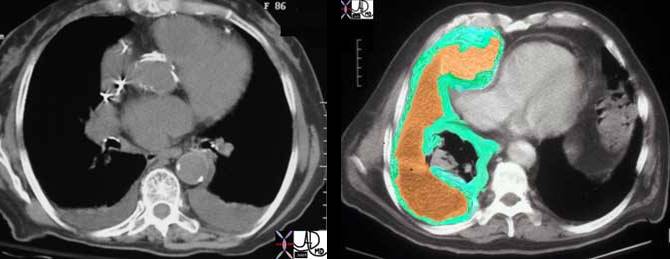
Right side: This CT scan represents a patient with a malignant tumor of the pleura called a mesothelioma. Note how thick the pleura are and how complex the associated effusion is. The lung is strangled and collapsed under the aggressive tumor. The right atrium is compressed by the tumor. Here the green overlay represents the tumor while the orange represents the loculated effusion.
Courtesy of: Ashley Davidoff, M.D.
Tension pneumothorax is an acute life threatening event, where there is not only collapse of the lung from the pneumothorax but also a pressure build up in the pleural space that causes the heart and great vessels to be compressed. This acute loss of support of the system is life threatening and if not treated by emergency decompression will lead to death. It would only take a small open-ended needle placed into the pleural space to decompress the tension and save a life. Sometimes when a patient suddenly collapses, and the condition of tension pneumothorax is a clinical consideration, physicians emergently put a small needle into the chest wall even before the chest X-ray confirms their clinical suspicions.
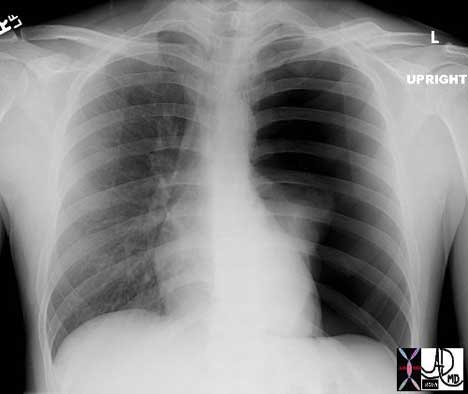
Courtesy of: Ashley Davidoff, M.D.
42525
Clinical Aspects
Acute inflammatory conditions of the pleura results in pleuritic chest pain. When the patient inspires the inflamed pleural surfaces rub against each other causing exquisite localized pain. The motion of the pleura becomes restricted by fibrinous exudates, and may cause a pleural “rub” – a squeaking sound caused by the restricted motion. As the pleural effusion enlarges the chances of contact of the two surfaces becomes less likely, and hence the pleural rub cannot be heard. Instead the large effusion compresses the adjacent lung causing a condition called “compressive atelectasis”. Percussion of the fluid results in dullness and auscultation results in amplification of sound called egophony.In the laboratory it is important to distinguish between a transudative effusion and an exudative effusion. This is done by evaluating the concentration of protein in the fluid. A transudate usually results from pulmonary congestion and contains limited protein and no fibrin, whereas an exudate is more complex and contains fibrin. Empyema is an infected pleural effusion and one of its hallmarks is the relatively low PH of the fluid.
Connective Tissues and Supporting Structures
There is a continuous network of connective tissue through the lung, starting from the hilum and ending at the pleura and sub pleural tissues. There are two basic systems, one known as the axial fiber system and the second the peripheral fiber system (Weibel). They are connected at the level of the alveolus by an intra lobular system of fine reticular fibers. Conceptually, it is easiest to consider the axial system starting at the hilum, and going in the direction of flow of the pulmonary arteries, and the peripheral system starting at the periphery and going in the direction of the pulmonary veins and lymphatics.
Connective Tissues and Supporting Structures: Axial Fiber System
The axial fiber system surrounds the bronchovascular bundle, which contains the bronchi, arteries, and their respective segmental and subsegmental progeny, up to the alveolar sacs. Thus the bronchovascular bundle starts at, and is anchored at the hilum, and ends within the acinus at the alveolar ducts and alveolar sacs. Presumably it is called the axial system because it courses along this major axis of the airways and pulmonary artery.
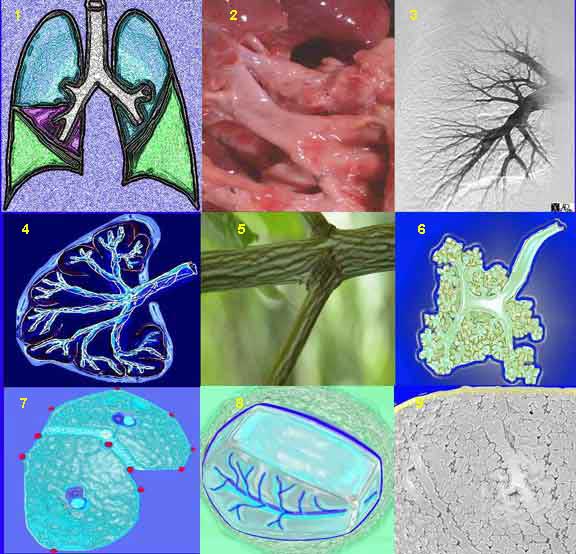
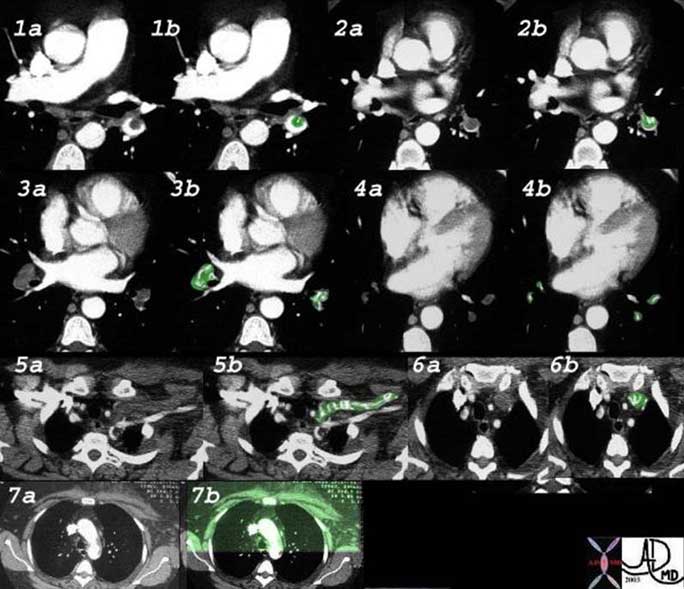
This is a collage illustrating the axial fiber system starting at the hilum, (1, 2) coursing along the pulmonary artery (3) and bronchovascular system, (3, 4, 6) surrounded by a basket of connective tissue (4, 5) extending into the polygonal secondary lobule (7, 8) and ending in the alveolar ducts and sacs (9).
Courtesy of: Ashley Davidoff, M.D.
Connective Tissues and Supporting Structures: Peripheral Fiber System
The second system, the peripheral fiber system, consists of the sub-pleural, pleural, and interlobular network and is closely linked to the veins and lymphatics (Weibel). One may think of it as starting in the subpleural space, extending into the pleura and then the interlobular septa, running along and supporting the veins and lymphatics and ending at, and anchored by, the hilum. Flow of both the veins and lymphatics is from the periphery toward the hilum. Presumably the origin of the term “peripheral system” relates to the concept that it functionally starts in the periphery.
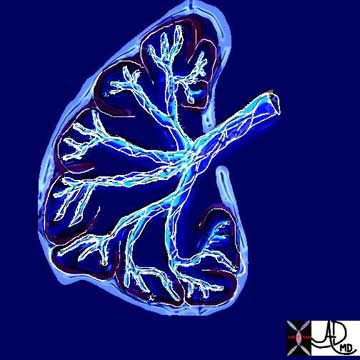
This is a diagrammatic representation of the basket of connective tissue support that surrounds the bronchovascular tree, (axial system) and the network that surrounds the secondary lobule extending from the pleura (peripheral fiber system).
Courtesy of: Ashley Davidoff, M.D.
Connective Tissues and Supporting Structures: Intralobular System
A less distinct system called the intralobular system consists of a fine reticulin meshwork surrounding the capillaries. It acts as the skeleton for the support of the alveoli. This network in effect connects the axial system and the peripheral system. In the end, all connective tissue systems are connected from head to toe. This is the fate and function of the connective system – they connect and support structures. Thus in the lung, identifying distinct axial, peripheral, and intralobular system is artificial, but is extremely helpful in the understanding of the structure and the changes that occur in disease.
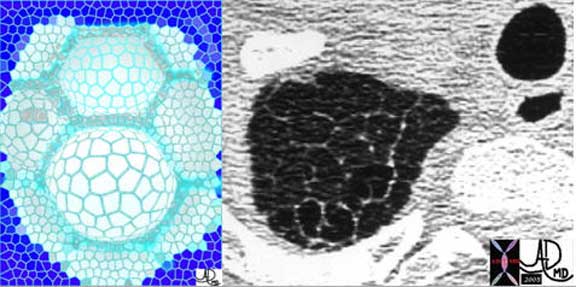
Courtesy of: Ashley Davidoff, M.D.
Connective Tissues and Supporting Structures: Applied Anatomy
Congestive cardiac failure with interstitial fluid accumulation results in a thickened interstitium. The chest X-ray will show effects of fluid accumulation in the interstitium. Within the peripheral system, this congestion will be reflected in subpleural edema, while at the alveolar level and axial level it will be reflected by Kerley B lines, and peribronchial cuffing.
Additionally, at the alveolar level the thickened interstitium inhibits gas exchange. Antifailure therapy which includes diuretics results in a decrease in the fluid and clearing of the interstitium. Other acute interstitial diseases include acute viral syndromes which are usually self limiting but their effect on the interstium with fluid and exudation can also affect gas exchange. Most acute interstitial diseases respond to medical therapy, or resolve, while the chronic interstitial fibrotic diseases are less likely to respond to therapy.Plain film examination of the lungs is remarkably sensitive to disease in the interstitium and pleural space. High resolution CT is able to show more exquisite detail of this part of the lung. We almost have a microscopic view of the lungs with both the plain film and CT. The plain film is also extremely helpful in the detection of pleural space disease, whether there is a pleural effusion or a small pneumothorax. Peribronchial cuffing is caused as a result of edema.
The clinical question surrounding a patient with shortness of breath or respiratory difficulty is such a common hospital and ICU scenario, and the diagnosis of alveolar disease (pneumonias) vs. acute interstitial disease (congestive cardiac failure) is very important. The distinction is made with the CXR and treatment with antibiotics vs. diuretics often rests on the findings of the CXR.
Courtesy of: Ashley Davidoff, M.D.
Blood Supply
In this section we discuss the blood supply to the lungs. There are two sets of arteries. The transport system needs a relatively small amount of oxygenated blood and the bronchial system is supplied by the bronchial arteries, while the exchange system has needs to supply a tennis court sized surface are and is supplied by the larger pulmonary arterial system.
Blood Supply: Blood Supply to the Airways
There are usually two bronchial arteries. The left artery arises from the aorta, and the right arises either from the 3rd intercostal artery (30%), with the 3rd intercostal as a common origin (intercostobronchial artery), from the thoracic aorta, or from one of the other proximal intercostal arteries. It is not uncommon to have 3 or 4 intercostal arteries.
The bronchial arteries supply the bronchi and the tissue of the lungs with oxygenated blood. As systemic vessels their pressure is at systemic levels, with a mean pressure that is 5-6 times higher than the mean pulmonary pressure (15 mmHg.).
The bronchial venous system drains into the right atrium and the azygos system.
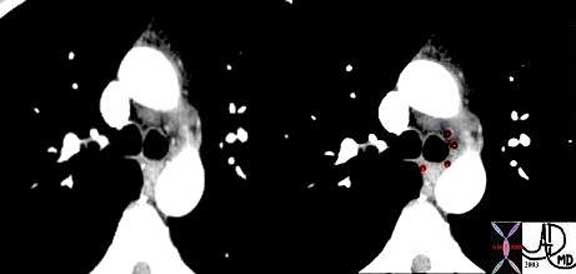
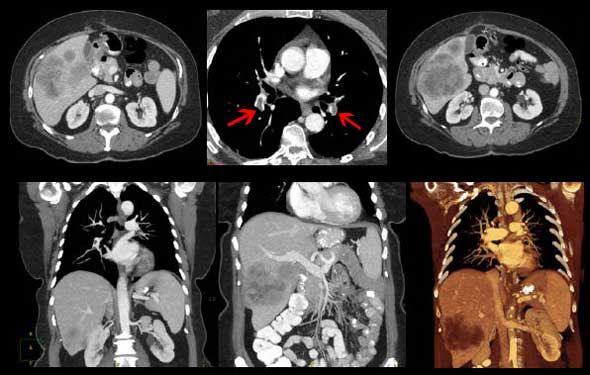
This CT in the early arterial phase shows four small arteries surrounding the carina, and represent branches of the normal bronchial arteries. The arteries are overlaid in red in the second image.
Courtesy of: Ashley Davidoff, M.D.
Blood Supply: Blood Supply to the Lungs
When we speak of an artery, we tend to think of a high pressured system with oxygenated blood. The common characteristic that defines them however is the fact that they carry blood away from the heart and toward an end organ. Hence the pulmonary artery, true to definition, carries blood away from the heart, but it is deoxygenated and is under relatively low pressure.
The pulmonary circulation receives more blood per minute than any other organ in the body since it receives the entire cardiac output from the right ventricle, together with a small component (1-2% of total pulmonary blood flow) from the bronchial arterial flow.
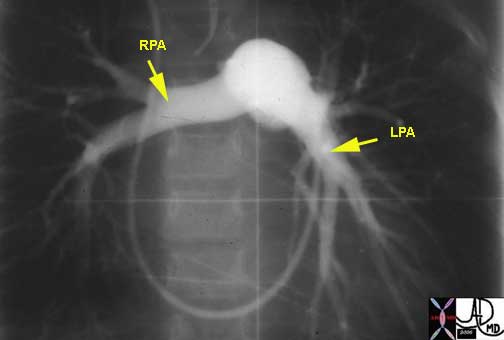
Courtesy of: Ashley Davidoff, M.D.
Courtesy of Ashley Davidoff, MD.
Blood Supply: Blood Supply to the Lungs Continued
The right ventricular outflow tract (RVOT) gives rise to the MPA which in turn divides into left and right pulmonary arteries. We have noted the difference in the anatomy of the right and left mainstem bronchi (right short and fat, left long and thin), and the similarity in the branching patterns of the distal bronchi and distal arterial system. While the RPA and LPA are similar in size they bear no resemblance to each other or to the mainstem bronchi.The direction that the two vessels are oriented is also completely different. The LPA courses directly posteriorly while the RPA courses directly laterally.
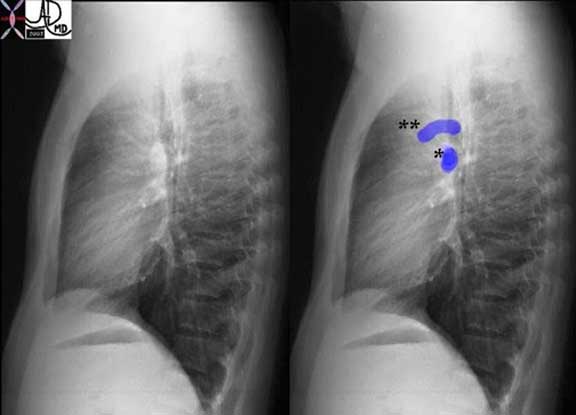
The pulmonary veins of the RLL can be seen running horizontally toward the LA while the PA’s to the RLL run a more vertical course.
Courtesy of: Ashley Davidoff, M.D.
The pulmonary veins of the RLL (red) can be seen running horizontally toward the LA while the PA’s to the RLL (blue) run a more vertical course.
Courtesy of: Ashley Davidoff, M.D.
The right pulmonary artery (RPA) takes almost a 140-degree turn from the main pulmonary artery. It rests on the top of the left atrium (LA) and has a straight shot in the direction of the midaxillary line. Thus on a lateral examination of the chest the LPA has the shape of an umbrella handle and the RPA is seen as an ovoid or rounded structure as we look down its barrel. The pulmonary veins are all inferior to the pulmonary arteries at the hilum.
Distal to the main pulmonary arteries, the branches follow the branching of the irregular dichotomous branching of the airways, and their morphology is similar till they enter the pulmonary lobule at the level of the respiratory bronchiole. At the capillary level, all the blood supplied by the pulmonary artery drains into the alveolar capillaries where they become oxygenated and then drain into the pulmonary venules within the interlobular septa, and finally back to the left atrium.
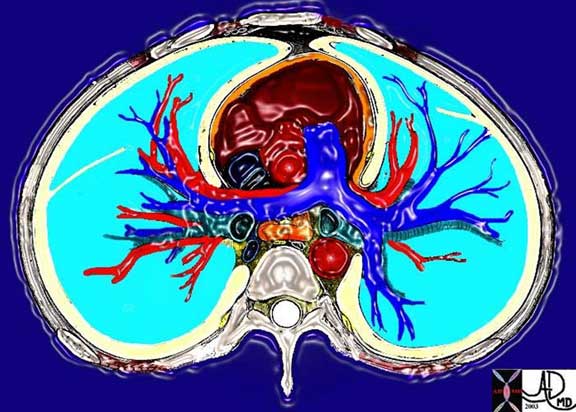
This cross sectional drawing shows the pulmonary artery in royal blue, pulmonary veins in red and the bronchi in teal. At the most central portion of each hilum there are usually 2 veins, one artery and one bronchus. This is because the length of the bronchus and artery prior to division is relatively long, while the confluence of the veins is close to the entrance into the left atrium. Thus the superior veins from the upper lobes are anterior and the veins to the inferior lobes are posterior.
Courtesy of: Ashley Davidoff, M.D.
Blood Supply: Applied Anatomy
The distinction between the arteries and veins on plain film examination is often difficult. At the level of the hilum, it is a little easier since the LPA is superior to the more easily identified left bronchus and the RPA lies under the similarly easily identified right bronchus. The confluence of the veins into the LA is always inferior to the pulmonary artery.
As we proceed beyond the hilum the artery can be identified, as long as its low-density air filled bronchus buddy is with it. As the structures move more peripherally the bronchioles get more difficult to see and the distinction between artery and vein becomes difficult.
In the lower lung fields the veins are horizontal as they course toward the left atrium while the arties have a more vertical course.
On CT, the same principles hold, but an added feature of the difference in branching angles of the vessels sometimes is helpful. Arteries usually branch at acute angles, and veins branch at 90° angle.
Pulmonary hypertension is characterized by enlarging arteries. The margins of the main arteries are usually quite distinct on the plain film. The lower lobe arteries should not measure more than 16mm in the male and more than 14mm in the female. They become blurred when there is interstitial edema, most commonly caused by heart failure.
Two of the common diseases of the pulmonary arteries include pulmonary embolism and pulmonary arterial hypertension. Pulmonary embolism has been an elusive diagnosis in the past and had been a commonly life threatening condition that was frequently missed. The advent of CT angiography with multidetector scanners has made the diagnosis much easier.
Pulmonary hypertension may be idiopathic, due to underlying chronic lung disease, or due to the less common congenital heart disease. In congenital heart disease pulmonary arterial abnormalities are common. In left to right shunts, including ASD, VSD, and PDA, there is an increase in flow in the pulmonary arteries due to the left to right shunt, with resulting increase in pulmonary pressure.
Another serious anomaly is a variety of hypoplastic growth abnormalities of the pulmonary outflow tract ranging from stenosis to total atresia.
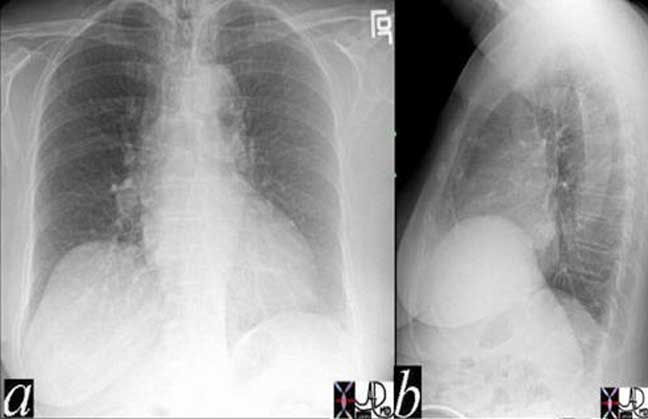
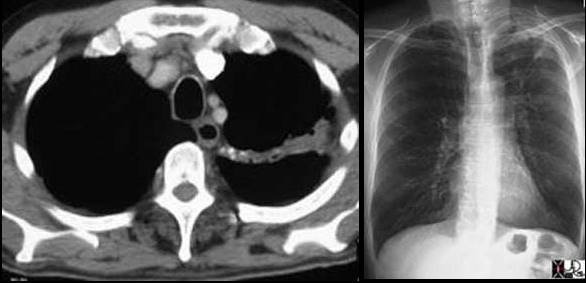
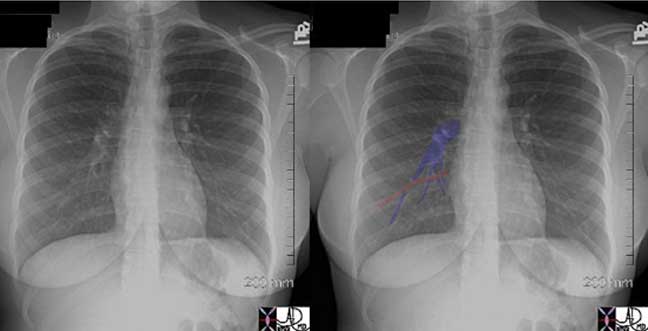
These two P-A chest X-rays show a normal cardiomediastinal silhouette on the left and an abnormally enlarged main pulmonary artery (MPA) andright pulmonary artery (RPA) on the right in this patient with pulmonary hypertension.
Courtesy of: Ashley Davidoff, M.D.

The lateral projection of this right ventricular angiogram reveals a case of severe pulmonary stenosis. The catheter enters the RV from the RA via the SVC. The RV inflow tract (purple) is hypoplastic. The vessels emanating and the RV inflow are coronary arteries that are filling in retrograde fashion and due to the suprasystemic pressures in the RV indicating severe pulmonary stenosis with pressures in the RV that probably exceed 100 mmhg. The infundibulum (right ventricular out flow tract (blue) is slightly narrow since it is hyperdynamic in an attempt to force the blood through the stenotic valve. The valve (green) is doming into the PA due to severe narrowing. The narrowing causes turbulence which causes the post stenotic dilatation.
Courtesy of: Ashley Davidoff, M.D.
The other arterial circulation to the lungs – the bronchial circulation, can also be the source of disease. In chronic disease states such as cystic fibrosis, and bronchiectasis for example, chronic increase flow due to the inflammation and infection occurs, and the combination of an infected and friable mucosa with enlarged arteries, is a cause for hemoptysis. The treatment of choice for recurrent hemoptysis is embolization of the bronchial arteries.
Venous Drainage
Venous drainage is usually via one major upper lobe and one major lower lobe pulmonary vein to each lung, but clinically significant variations do exist. Segmental and subsegmental veins and venules are the equivalent subdivisions of the veins. The capillaries surround the alveoli.
The pulmonary veins drain the lungs of the blood supplied by the pulmonary artery, and the bronchial veins drain the blood from the areas supplied by the bronchial artery. Whereas the pulmonary veins contain oxygenated blood, the bronchial veins contain deoxygenated blood. 98-99% of the blood in the lungs is in the pulmonary arterial – venous circulation while the remaining blood is in the bronchial circulation.
The bronchial veins have a deep and a superficial system. The deep bronchial veins drain either into the pulmonary veins or into the left atrium. The superficial bronchial veins drain the extra pulmonary bronchi, visceral pleura, and hilar lymph nodes and drain into the azygous system on the right side and the superior intercostal vein or the hemiazygous system on the left.
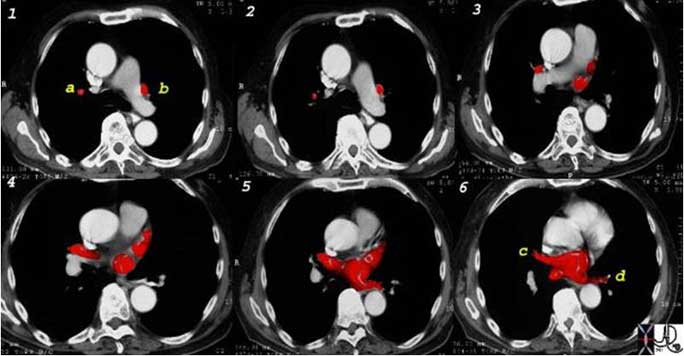
This is a collage showing the CT appearance of the pulmonary veins as they converge onto the left atrium. Note the vertical course of the right superior (a) and left superior veins (b) in image 1, where they appear as rounded to ovoid structures. In image 6, the right inferior (c) and left inferior (d) are seen as tubular structures since their course is more horizontal.
Courtesy of: Ashley Davidoff, M.D.
Pulmonary Veins
There are usually 4 pulmonary veins. They are the superior and inferior right pulmonary veins and the superior and inferior left pulmonary veins.
The right superior pulmonary vein drains the right upper and middle lobe. The two veins join to form the most anterior structure in the hilum. The left superior pulmonary vein drains the left upper and lingular segments. The inferior veins drain the lower lobes.
The veins are the shortest of the three major vessels originating in the hilum. The longest structures are the bronchi and second to them are the pulmonary arteries. This has implication in cross sectional imaging where all these structures converge. Therefore when the central areas are viewed, there will be a single bronchus and artery, but usually two veins on either side. The upper veins will be anterior to the bronchus and artery and the lower veins will be posterior and inferior.
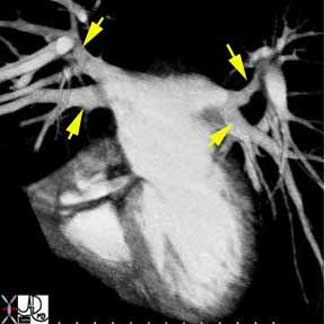
Courtesy of: Ashley Davidoff, M.D.
Venous Drainage – Applied Anatomy
Diseases of the pulmonary veins are most commonly secondary to left sided heart disease where an elevated left ventricular or left atrial pressure is reflected in the pulmonary veins. There are a series of congenital anomalies of the pulmonary veins including partial and total anomalous venous return.
Total anomalous pulmonary venous return (TAPVR) can occur with the final destination either above the diaphragm or below. Sometimes, the TAPVR below the diaphragm connects to primitive venous origins of the hepatic circulation so that pulmonary venous return is to the sinusoids of the liver. This condition as an untreated anomaly, cannot sustain life since the pressure of the oxygenated blood in the sinusoids is close to zero, and there is therefore no driving force to allow it to traverse the sinusoids. Hence there is a functional obstruction.
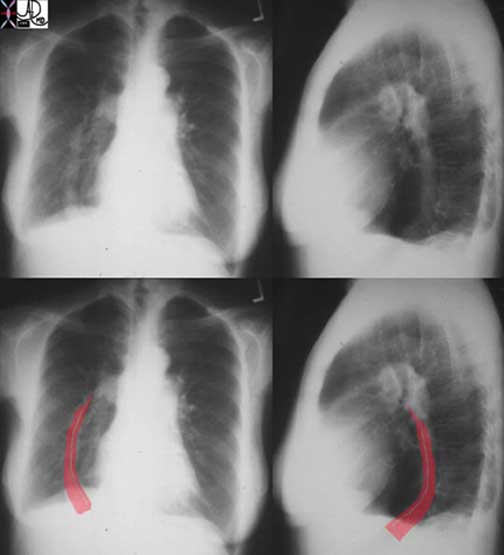
Courtesy of: Ashley Davidoff, M.D.
Nerve Supply
There are three types of nerve fibers that connect the lungs to the autonomic nervous system. Autonomic afferent fibers travel to the vagus nerve via the pulmonary plexuses, originating in the airways and the lungs. They transmit information from stretch receptors. Parasympathetic efferent fibers are all contained in the vagus nerve. They carry motor impulses to the airways and lungs which cause bronchiolar muscle contraction, gland secretion, and vasodilatation. The third group is the sympathetic efferent fibers which relax bronchial muscles, inhibit glandular secretion, and cause vasoconstriction.
The walls of the bronchi and bronchioles are innervated by the autonomic nervous system. There are b2 adrenergic receptors in the bronchial epithelium and smooth muscle. The b2 receptors are responsible for bronchodilation and secretion. The a1 adrenergic receptors, on the other hand, inhibit secretion.
Lymphatic Drainage
Two sets of lymphatic vessels drain the lung of lymph. A subpleural lymphatic network collects the lymph from the peripheral lung tissue and drains it along the veins leading toward the hilum. There is a deeper lymphatic system that originates around the bronchi and the bronchioles. The deep system joins the lymphatics around the larger bronchi and pulmonary arteries and they finally enter the mediastinum where they join the bronchomediastinal trunks. The final common pathway for all the lymphatic is via the thoracic duct which enters the left subclavian vein.
Most of the visible lymph nodes are within the hila and mediastinum. However, there are lymph nodes that lie close to the periphery of the lung. These are relatively small measuring approximately 2mm in diameter. They become larger towards the hila, reaching diameters of between 5 to 10mm.
The mediastinal nodes have been divided into 4 main groups:
Superior mediastinal
Aortic
Inferior mediastinal
N node
Within these groups there are 14 nodal stations. These 14 stations have been given both names and numbers to aid in the classification and staging of disease.
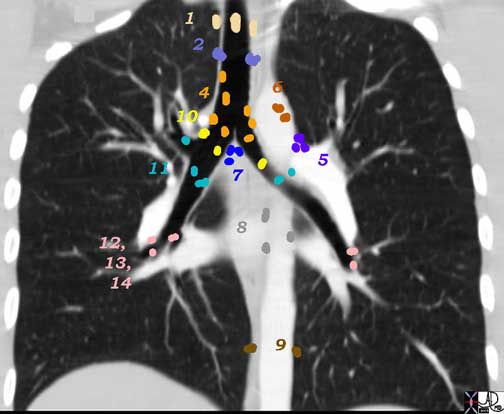
1 Highest Mediastinal Nodes
2 Upper Tracheal Nodes
3 Prevascular and Retrotracheal Nodes
4 Lower Paratracheal Nodes
Aortic Nodes
5 Subaortic Nodes (A-P window)
6 Paraaortic Nodes (Ascending Aorta or Phrenic)
Inferior Mediastinal Nodes
7 Subcarinal Nodes
8 Paraesophageal Nodes
9 Pulmonary Ligament Nodes
N Nodes
10 Hilar Nodes
11 Interlobar Nodes
12 Lobar Nodes
13 Segmental Nodes
14 Subsegmental Nodes
Ashley Davidoff MD
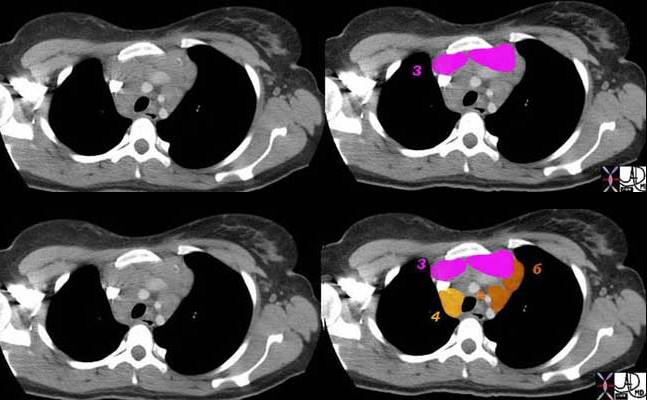
3 = Prevascular
4 = Lower Paratracheal
6 = Paraaortic Nodes
Ashley Davidoff MD
Lymphatic Drainage: Subpleural System
Certain diseases have a predilection for the lymphatic system at the subpleural level including sarcoidosis. On the other hand, diseases such as lung carcinoma have a predilection for the deep and central nodal system. Since the systems do connect and are both usually involved, it is imperative to evaluate both systems.
Sarcoidosis and sarcoid nodules in particular, specifically seek out the lymphatics of both the subpleural and the deep systems. Thus when nodules or focal changes are identified on the pleural surfaces, including the fissures, interlobular septa, and bronchovascular bundles then sarcoidosis is a prime suspect. Pleural effusions on the other hand are distinctly uncommon in sarcoidosis.(1-4%). In lymphangitic spread of disease, malignant cells get bundled into the lymphatics causing obstruction and reducing pulmonary capacity. Thickening of the interlobular septa is a characteristic finding in these cases. In anthracosis, lymph nodes and lymphatics become filled with carbon colored soot.
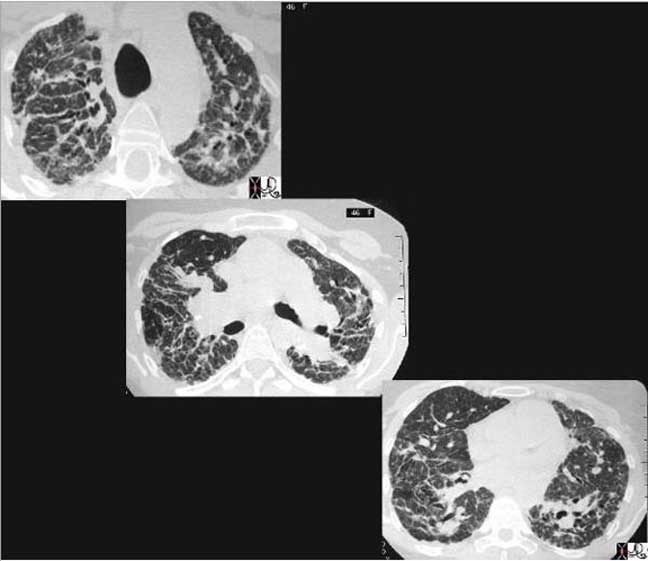
This cross sectional series of three CT images shows end stage sarcoidosis characterized by marked thickening along the lymphovascular bundles. The first image in the upper left shows marked thickening of the interlobular septa caused by granulomatous changes along the lymphatics.
Courtesy of: Priscilla Slanetz, M.D.
Lymphatic Drainage: Mediastinal Nodes
In general, although we often measure the size, and specifically the short axis size of the nodes to determine the presence of disease, we understand that this is a fairly inaccurate method with low specificity . A short axis of more than 10mm implies a pathologically involved node. Often the large node may be reactive and may not contain malignant disease. PET scanning has been an important advance to aid in the distinction between reactive and malignant lymphadenopathy.
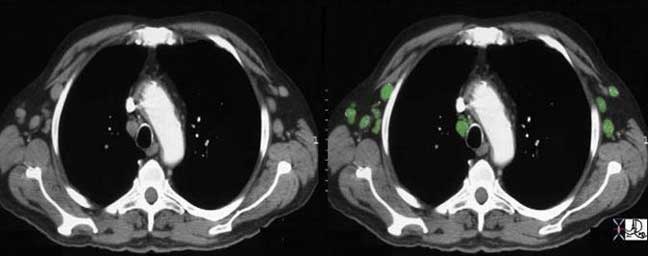
Courtesy of: Ashley Davidoff, M.D.
Courtesy of: Ashley Davidoff, M.D.
Aging of the Lungs
While the number of bronchi one has at birth is the same as the number in old age, the number of alveoli increases until the age of about 8 years. Thereafter the alveoli increase in size until about 18 years. As the lungs progress through age there is a mild, functionally insignificant increase in size due to loss of elasticity. The respiratory bronchioles and alveolar ducts increase in size in middle age, but there is no destruction of the walls of the alveolar septa. Thus in old age there is a mild increase in the size of the lungs but without concomitant destruction of lung tissue that is seen in emphysema. There is glandular hypertrophy and progressive calcification of the cartilage of the medium sized bronchi.
The Lung and Disease
The lungs are closely connected to the immediate environment and hence they are directly exposed to any and all pathogenic entities in the environment, including biological and non biological elements.
Bacteria and viruses are universal and therefore are common pathogens and affect everybody. Immune status is key to survival from these infections, and so the young and the old, and those with immune deficiencies are particularly vulnerable to these unseen enemies. Other diseases such as the dust diseases and pneumoconiosis, occur in isolated work related environments, but there are some diseases, like bird fancier’s disease that have both acute and chronic manifestations due to exposure to the antigen which is found in the droppings of birds – most commonly pigeon’s but also from domestic birds like budgerigars (small parakeets).
The Lung and Disease: Infection – Pneumonia
Infections to the lungs are caused by a host of organisms and most commonly are viral or bacterial in origin. Anatomically they can affect the airways causing epiglottitis, pharyngitis, tracheitis, bronchitis or bronchiolitis. The infection, on the other hand may affect the alveolar components of the lungs causing lobar or segmental pneumonia, or the interstium of the lungs causing an interstitial pneumonitis. Since the airways and alveoli by definition are connected the involvement of one part does not preclude the involvement of the other, though certain organisms have a predilection for certain parts of the respiratory epithelium. Additionally an infection may start out as viral and progress and become complicated by secondary bacterial infection. Within the lung, interstitial infections (usually viral), will appear on a chest X-ray with a linear pattern while the classical bacterial pneumonia will present as a consolidation.
The most common form of pneumonia in the population is viral in origin while the most common bacterial pneumonia is streptococcal in origin.
There are some characteristic features about the bacterial pneumonias that are sometimes helpful in the differential diagnosis, though it is often difficult to make an organism specific diagnosis based on imaging. In general the bronchopneumonias affect infants’, young children and the elderly and are caused by streptococcus pneumonia and affect airways and alveoli contiguous to the larger bronchioles. Bronchopneumonia is characterized by peribronchial thickening, lobular nodules of patchy airspace disease, which may progress to lobar disease. Lobar pneumonia affects young adults and presents as a consolidated mass affecting segments, lobes, singly or at multiple locations. Lobar pneumonia is also usually caused by Streptococcus pneumoniae (aka pneumococcal pneumonia) (Todar’s On Line Text of Bacteriology), and Klebsiella. It is primarily a disorder of the alveoli resulting in segmental or lobar consolidation with the air bronchogram being characteristic.
Klebsiella infection tends to occur in the upper lobes and causes significant swelling of the lung so that bulging fissures may be seen. It also can cause cavitation.
Legionella has a tendency to affect the lower lobes.
Mycoplasma has a tendency to affect the lower lobes.
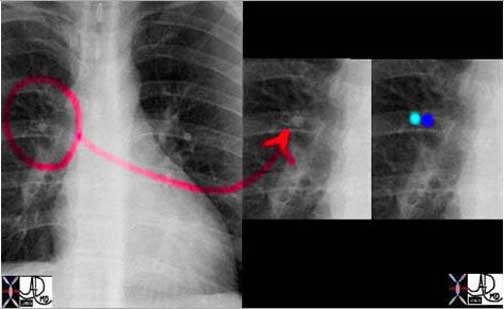
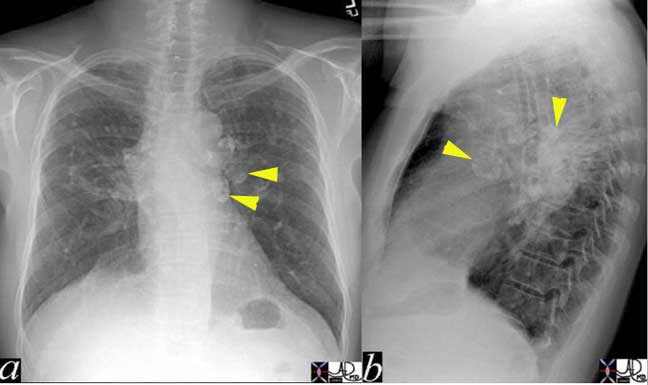
The CXR in lobar pneumonia usually shows a consolidation exemplified by an air bronchogram. In this case an infiltrate (yellow arrowheads) is seen in the right middle lobe (a, b) which resolved 4 weeks later, after antibiotic therapy (c, d).
Courtesy of: Ashley Davidoff, M.D.
The Lung and Disease: Viral Pneumonia
The most common agents causing viral pneumonia are influenza A, respiratory syncitialk virus (RSV) and paraifluenza 1,2,3.
Radiological findings are classically of a linear interstitial nature but they may also show alveolar changes such as consolidation. Pleural effusion and peribronchial thickening are associated findings.
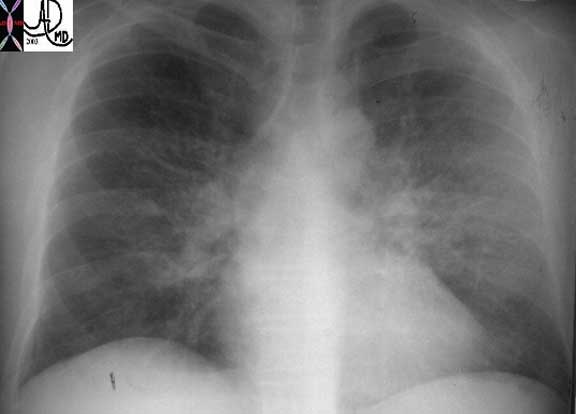
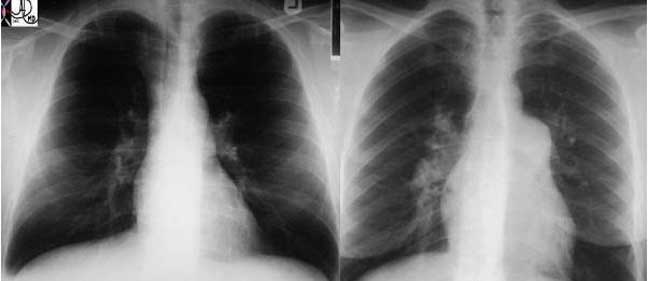
This plain film of the chest (CXR) shows classical reticular interstitial process pneumonia. Note this pattern is more linear and represents an interstitial pneumonitis.
Courtesy of: Priscilla Slanetz, M.D.
The Lung and Disease: Pneumonia in the Immunocompromised Host
There is a high incidence of pneumocystis pneumonia (PCP) in patients with AIDS. Other organisms such as gram negative bacilli, staphylococcus, aspergillus and cytomegalovirus are also well known pathogenic organisms in this population of patients. (eMedicine – Fernandes). Up to 45% of all HIV respiratory infections are bacterial in origin occurring at any CD4 count. As the CD4 count decreases the incidence increases, particularly when the CD4 count is less than 200 cells/mm 3.
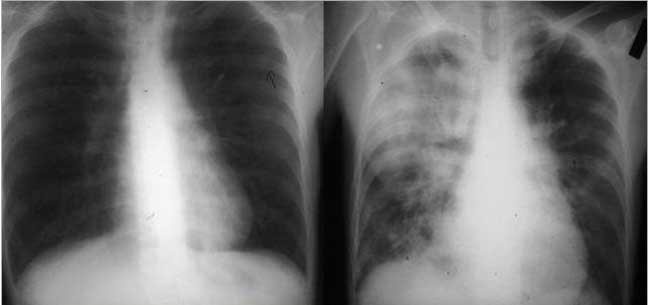
These images are from a patient with immune deficiency and they show a progression of disease from near normal CXR to a consolidated right lung dominating in the right upper lobe. Pneumocystis was identified as the incriminating organism. The most frequently described appearance on chest radiograph is a diffuse, bilateral interstitial infiltrate, consisting of fine-to-medium reticular interstitial change.
Courtesy of: Ashley Davidoff, M.D.
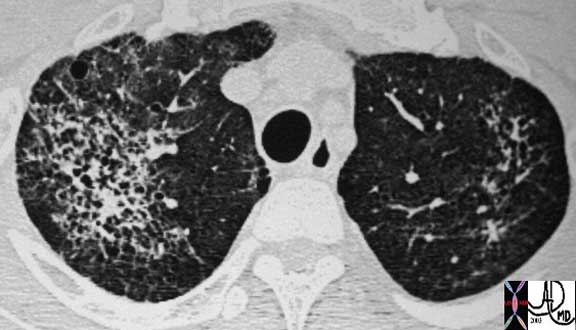
This is a CT of a 44-year-old patient with HIV and with PCP infection. In this instance there is diffuse involvement of thick walled irregular cavities associated with a thin walled cyst seen anteriorly in the left lung. The most frequently described appearance on chest radiograph is a diffuse, bilateral interstitial infiltrate, consisting of fine-to-medium reticular interstitial change.
Courtesy of: Ashley Davidoff, M.D.
The Lung and Disease: Mycoplasma Pneumonia
Mycoplasma is a genus of bacteria that lacks cell walls. When it affects the respiratory system it presents with fever, headache, malaise, and anorexia. The white cell count is usually normal but may show a left shift. Cold agglutinins are detected in 50-70% of patients and the diagnosis is made with a PCR assay of a nasopharyngeal swab or serologic testing.. The CXR has a variable appearance and can present both with airspace disease in segmental or lobar fashion (45-60%) or with an interstitial pattern of nodular or reticular type (15-50%). Pleural effusions are relatively uncommon (<20%). On CT they may present with a bronchioloitis with centrilobular nodules, alveolar consolidation, interstitial thickening of the axial interstitium or of the interlobular septa.
Most infections resolve and the morphology of the lung returns to normal. With repeated infection and or untreated disease, there may be permanent and ongoing structural damage to the lung or the airways and entities such as bronchiectasis may result.

This CT image shows a hyperinflated and large right lung, with volume loss and cystic change in the left lung. Note the trachea is pulled to the left by the contracted left lung, and is pushed by the hyperinflated right lung. The disease may be as a result of previous severe or untreated pulmonary infection.
Courtesy of: Ashley Davidoff, M.D.
The Lung and Disease: Inflammatory Diseases
Inflammatory diseases such as the collagen vascular diseases, interstitial pulmonary fibrosis, and sarcoidosis are idiopathic and slowly advancing. Understanding of the causes of these diseases and the underlying pathology is one of an inflammatory process affecting the airways, vessels, connective tissues or the parenchyma.
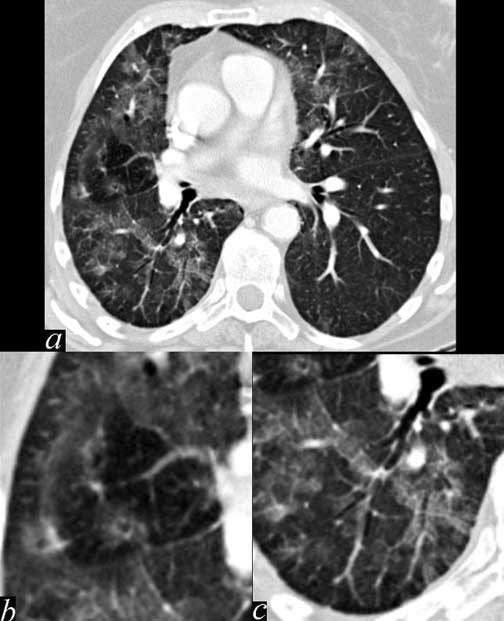
This patient demonstrates significant patchy bilateral lung disease that dominates in the right lung. (a) In image b, there are ground glass changes (areas of increased density) areas of mosaic perfusion (areas of decreased density), and peribronchial halos of edema. In c there are small air bronchograms suggesting alveolar involvement. These findings are characteristic of an acute inflammatory process such as an acute allergic pneumonitis.
Ashley Davidoff MD
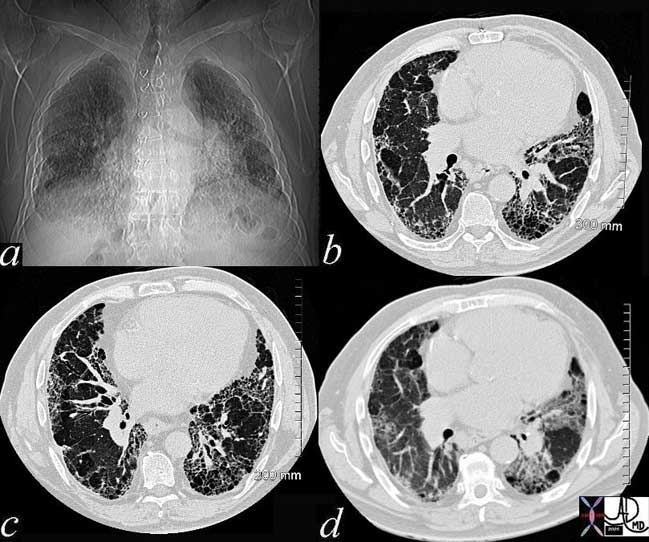
Ongoing inflammatory disease of the interstitium will result in pulmonary fibrosis typified by the interstitial and reticular (net like changes) seen on the plain film (a) and the “honeycombing effect exemplified in the high resolution images (b, c) and conventional image in (d).
Courtesy of: Ashley Davidoff, M.D.
The Lung and Disease: Malignant Disease and Other Growth Abnormalities
The malignant diseases of the lungs commonly have a cigarette smoking habit to blame, and the aggressive, space occupying, spreading nature of the disease usually has a negative outcome as we struggle with different and new therapeutic regimens to prolong life. The small cell carcinomas of the lung are usually bulky and aggressive, respond dramatically to therapy but then tend to recur. The non small cell cancers have surgical options and a potential cure. The potential for cure rests on the staging of the disease.
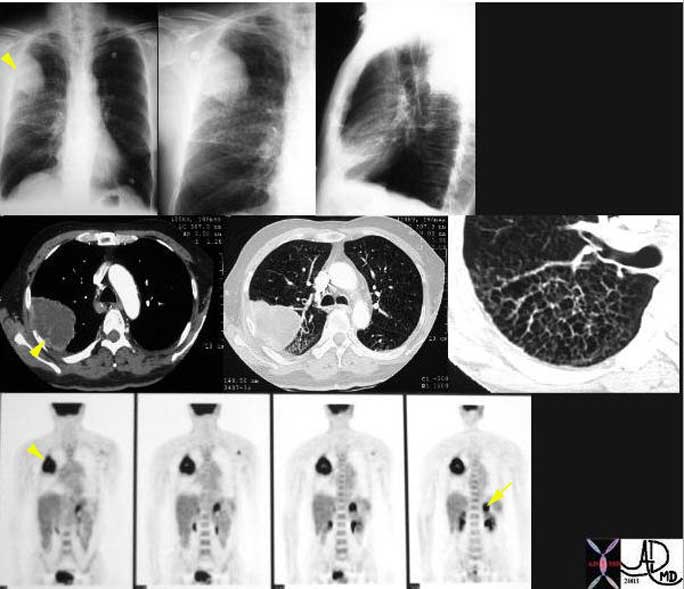
This is a patient with lung carcinoma presenting with a large mass in the right upper lobe (arrowheads) with a lymphangitic pattern, adrenal metastasis and a PET positive scan for the mass and for the left adrenal gland (arrow).
Courtesy of: Ashley Davidoff, M.D.
The Lung and Disease: Congenital Disease
Congenital and genetic disease of the lungs is not common in the adult population but sporadic cases do occur. Progress in the management of patients with cystic fibrosis has resulted in an enlarging adult population with this disease which was previously fatal in childhood.
The Lung and Disease: Mechanical Diseases
Emphysema can be considered a mechanical disease of the lungs since the loss of elasticity of the lung tissue that occurs together with the destruction of tissue results in mechanical disadvantage. As a result air cannot be moved efficiently and there is an increase in air that is trapped in “dead” space and which cannot be efficiently moved to the capillaries for exchange nor efficiently removed. Emphysema is a major cause of morbidity and mortality in the smoking population.
Asthma, another common disorder is an obstructive disease that inhibits the transport of air to and from the lungs as well.
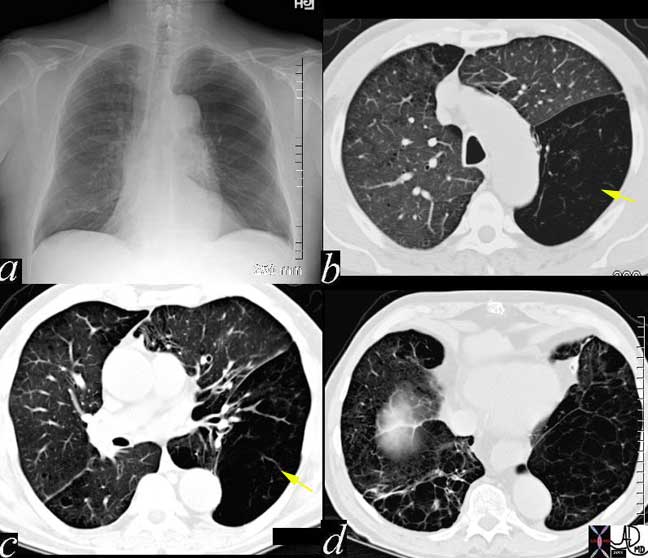
In this patient the lower lobes (yellow arrows) and particularly the LLL is affected by air trapping probably related to bronchiolar disease with resulting ball valve effect. In this entity, air can enter and cannot leave the alveoli and hence there is progressive inflation of the affected segments.
Courtesy of: Ashley Davidoff, M.D.
The Lung and Disease: Pulmonary Embolism
The advent of multidetector CT has brought about a revolution in our ability to diagnose this relatively common and often fatal disorder. Our ability to diagnose pulmonary embolism (PE) with CT requires meticulous care to the technique and bolus timing since contrast volume limitations precludes a second injection because of the nephrotoxicity of the contrast.
This elderly woman presented with acute respiratory difficulty with swelling of her left upper limb. CT shows extensive pulmonary embolic disease to the lower lobes from second order branches to the tertiary branches (1 – 4 with green overlay) and evidence of thrombosis of the left subclavian vein (5, 6). Swelling over the anterior chest including the pectoralis muscles is evident in 7.
Courtesy of: Priscilla Slanetz, M.D.
The Lung and Disease: Pulmonary Embolism with Infarction
Although pulmonary embolism is a common pulmonary infarction it is distinctly uncommon because the lung receives blood supply from the bronchial as well as the pulmonary artery and can also be oxygenated directly from the alveoli.

In the case to the right a wedge shaped infarction is seen in the periphery of the right lung (purple). Both vessels coursing toward it are occluded by thrombus (red and green). The patient is in heart failure with cardiomegaly and small bilateral effusions noted.
Courtesy of: Ashley Davidoff, M.D.
The Lung and Disease: Heart Failure
Heart failure is a very common entity and despite the progress with MDCT, the CXR is still the most sensitive imaging technique for this entity.
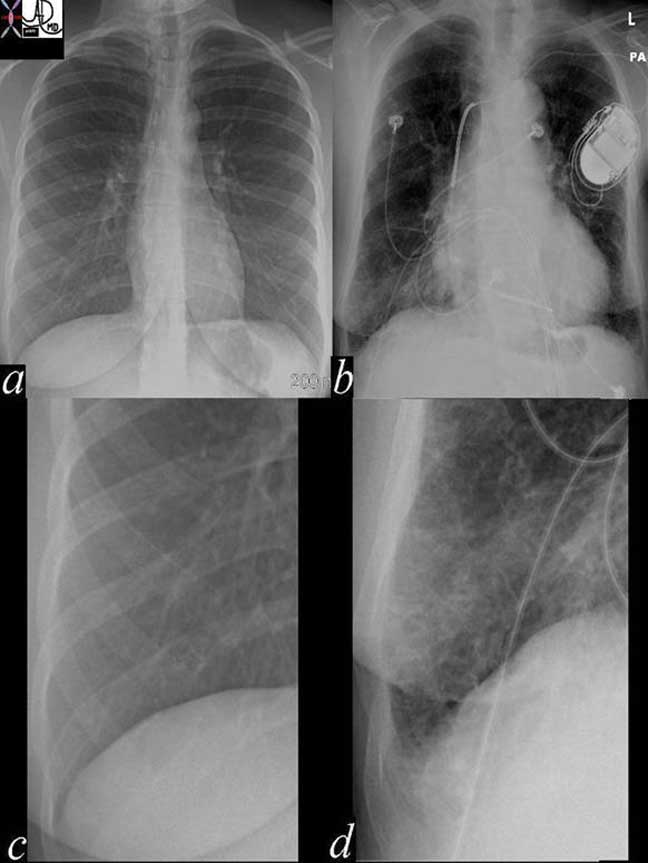
In the images above the normal example is noted in image (a) with the normal costophrenic angle noted in (c). Image (b) reveals a patient in congestive heart failure with an enlarged heart and prominent interstitial changes in the costophrenic angles called Kerley B lines that characterize thickened and congested interlobular septa (d).
Courtesy of: Ashley Davidoff, M.D.
Imaging of the Lungs – Technical Aspects
The plain film chest X-ray (CXR) is still the most commonly used radiological examination and the PA and lateral view when possible provide the most information.The PA is taken with the anterior portion of the chest on the cassette, with tube being 72 inches away minimizing divergence and so the heart is relatively small. When the projection is AP, the source is only 40 inches away (i.e. X-rays enter from anterior). Under these circumstances, divergence is a factor and the heart and mediastinum become relatively magnified.
Deep inspiration on a routine CXR gives us the best opportunity to study all the structures we have discussed above. The mediastinum contains soft tissue structures while the lungs are lucent. The lungs therefore require less penetration of X-rays and the mediastinum and the heart require more. The left lower lung can be invisible on the CXR if the film is underpenetrated and so it is better to be over than underpenetrated.
Important anatomic structures relevant to evaluation of the P-A view, are the interfaces of the lungs with the mediastinum, the ability to evaluate the left lower lung field, the ability to clearly see the bronchovascular bundles.
The advent of CT scanning with a superior gray scale, and with digital technology, has helped solve this problem, so that we are able to window in to the gray scale of the specific part being imaged. We can, for example, look specifically at the lungs using the gray scale and window level appropriate for the lung, (lung windows) for the mediastinum (mediastinal windows) or for the bones (bone windows). CT enables us to visualize subsegmental bronchi and bronchioles in normal conditions.
Computed Tomography (CT)
CT technique employed in the examination of the lungs is based on the clinical indication. There are a few techniques that are utilized:
Conventional Routine Examination without Contrast: 3-5mm thickness slices
Conventional Routine Examination with Contrast: 3-5mm thickness slices
High Resolution CT: 0.75-1mm thickness slices
Rule Out Pulmonary Embolism: 0.75-1mm thickness slices
Conventional technique with 64 slice multiple-row detector (MDCT) scanner uses 3mm thickness slices with detector collimation of 0.6mm. If contrast is needed then 120cc’s of Omnipaque 350 is used at an injection rate of 1cc/second with a scan delay of 60 seconds.
For high resolution imaging using the 64-slice MDCT scanner, a slice thickness of 3mm and 0.75mm are acquired with collimation width of 0.6mm. Contrast is not generally used.
If there are pulmonary changes at the posterior dependant portions this may be due to passive atelectasis due to poor movement of air and prone imaging may help inflate these regions and provide a more accurate evaluation of the disease process.
For a pulmonary embolism (PE) study on the 64 slice MDCT scanner, the slice thickness used is 0.75mm, collimation is .6mm, and 100-120ccs of Visipaque 320 is used with a scan delay of 25-30 seconds or patient specific bolus timing or tracking can be employed.
Conventional 3 – 5mm slice thickness imaging is used for the evaluation of pneumonia, pleural effusion, lung nodule follow up for example. The addition of contrast in these studies is based on the need to identify lymphadenopathy and other structures in the mediastinum. This is particularly necessary in the evaluation of lung carcinoma and in most cases of inflammatory diseases of the lungs including sarcoidosis, which has a predilection for lymph nodes but is also required in imaging the patient with lymphoma. If empyema is a clinical concern then contrast is helpful to determine if there is rim enhancement of the pleura a sign indicating inflammation in the pleura. Contrast is also essential if there is clinical concern for an arteriovenous malformation.
High resolution imaging (0.75 -1mm slice thickness) is utilized when interstitial lung disease is a clinical or radiological consideration. As conventional MDCT gets down to the 3mm thickness slice range we are close to high resolution of yester year, but the 1mm collimation still offers significant advantage in spatial resolution. It is imperative that the patient is able to lay still and breathhold for this technique.
Timing the bolus presents a technical challenge since the cardiovascular status in the ill is so variable. One may suspect that bolus tracking would have been the answer to our problems but in fact it does not give us the consistently well timed bolus. We aim for a density of contrast in the pulmonary artery of about 90 HU.
Several methods of bolus administration have been employed. A uniphase injection with a rate of 4mls/sec has been suggested as the preferred technique. (Bae et al Radiology, 2000, 216:872-880) If the injection duration is prolonged then recirculation of contrast causes an additive effect of existing contrast and increases the density in the artery. Bolus tracking would start the injection relatively early and cumulative effect would not be accomplished. One could argue that the threshold level could be set higher but in the face of poor cardiac output the higher threshold may not be reached and the scan would never be triggered. A scan delay of 25 – 30 seconds usually allows for optimal technique in most patients and individual tailoring based on age and hemodynamic status must always be considered.
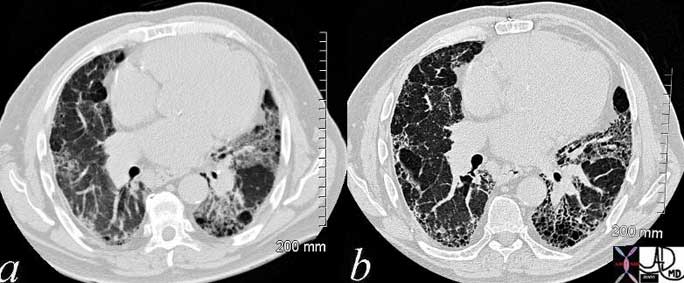
The clarity of the structural changes as seen on the high resolution image (b) compared to the conventional image (a) is obvious in this example of pulmonary fibrosis and IPF with honeycomb changes.
Courtesy of: Ashley Davidoff, M.D.
Technical Aspects: PET Scanning
The advent of PET scanning has revolutionized the diagnosis and staging of lung cancer. The technology is based on the avidity of tissue for fluorodeoxyglucose (FDG) which is tagged with fluorine 18. Metabolically active tissues, exemplified by malignant tissue as well as some inflammatory diseases have avidity for the fluorodeoxyglucose. Lesions usually have to be more than 7mms in order for them to be seen by PET imaging. Some carcinomas such as the bronchiolar alveolar carcinomas do not take up the FDG and are notoriously negative. Thus a negative PET scan does not exclude the diagnosis of malignancy, and a positive PET study does not definitely imply malignancy.
Imaging the Chest
From the radiological point of view, X-rays used in plain chest X-ray or CT scan, move almost unimpeded through the lungs, and present as a radiolucency or blackness on the image. On the other hand, the mediastinum serves as a moderate barrier to X-rays, and the ribs and spinal column present an even greater barrier. The tissues of the chest vary significantly in the degree to which they absorb or reflect X-rays. This difference presented a challenging problem to radiologists and physicists over the years, particularly related to technical factors that would optimize imaging of the mediastinum and heart on the one hand, and the lungs on the other. The advent of CT scanning with a superior gray scale, and with digital technology, has helped solve this problem, so that we are able to window in to the gray scale of the specific part being imaged. We can, for example, look specifically at the lungs using the gray scale and window level appropriate for the lung, “lung windows” for the mediastinum “mediastinal windows” or for the bones “bone windows”.
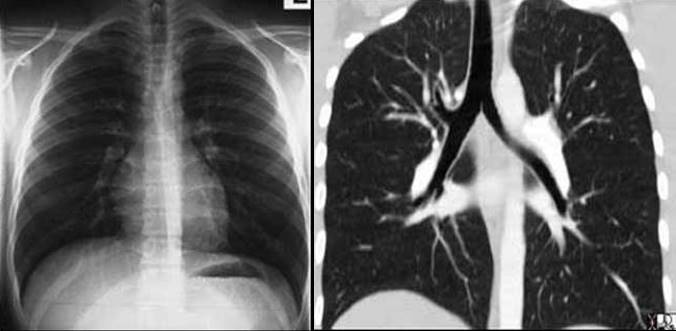
Courtesy of: Ashley Davidoff, M.D.
Imaging of the Chest: Imaging – Chest X-ray
Why do we see structures on X-ray? We are able to define two side by side structures if they have contrasting X-ray absorption characteristics and hence densities. On a plain film of the chest for example, the trachea and main bronchi are usually visualized because the air filled trachea is surrounded by relatively thick soft tissue walls and other soft tissues of the mediastinum creating the necessary density differential to enable distinction. The air in the visible mainstem bronchi is surrounded and separated from the surrounding air filled parenchyma and by relatively thick walls of soft tissue density and this contrasting density allows the mainstem bronchi to be seen. As we progress to the smaller bronchi, usually after the fourth order of branching, the soft tissue walls become too thin to create an air-soft tissue interface and hence we cannot resolve nor see them.
Almost 90% of the lung is air and only about 10% is soft tissue, interstitium and blood. The dominant density of the lungs is therefore black or lucent. The soft tissues of the lung, including the blood vessels and the connective tissue (also called the interstitium), impede the X-ray, resulting in a gray or soft tissue density. The interface of lucent air (black) against a soft tissue background of the interstitium (gray) provides a sharp and contrasting interface of density, allowing an almost microscopic view of the lungs. If the pressure in the capillaries becomes abnormally high, for example more than 25 mmHg, fluid starts to leak into the interstitium. The clarity of the interstitial markings becomes reduced and blurry. The radiologist is able to imply pathophysiological changes based on the fuzziness of the blood vessels and can approximate the pressure in the capillaries. This change is best appreciated on a plain film of the chest. The plain chest film has stood the test of time, proving its value since Roentgen’s discovery in the late 19th century.
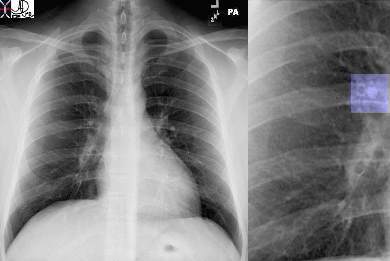
Courtesy of: Ashley Davidoff, M.D.
42464c02
Most disease states replace the air of the lungs with fluid or soft tissue, and appear as increasing densities on the CXR. In pneumonia the alveoli become filled with pus or fluid. The air in the lung parenchyma is replaced by soft tissue elements. If there is persistence of air in the smaller airways, they will now be visualized, since the air in the airways is now in contrast to the surrounding density of the fluid filled parenchyma.
Imaging of the Chest: The Secondary Lobule Revisited
CT with a wider gray scale enables us to visualize subsegmental bronchi and bronchioles both in normal conditions and under various pathological situations. The secondary lobule is a unit of the lung that is enclosed by an incomplete capsule called the interlobular septum, best developed in the periphery of the lung and specifically at the lung bases and the apex, and incompletely formed in the rest of the lung. In the evaluation of lung diseases the diagnostic dilemma starts with the anatomic question as to which part of the lung is involved with disease – the so called anatomical differential diagnosis. Is it the tubular airways, the alveoli, the interstitium, the arteries, the lymphatics or the veins? Since we know that the center of the secondary lobule contains the airway and the arteriole, the matrix contains the alveoli, and the periphery contains the interlobular septum, the lymphatic and the vein, we always search for the polygonal structure or vestiges of it to help us hone down on the structure that is involved.
Imaging of the Chest: Small Airway Disease
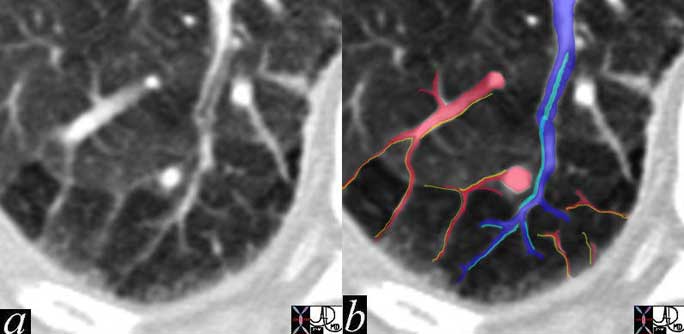
In this remarkable CT we were able to identify a few secondary lobules at the periphery of the lung that have a rectangular shape in this instance. The branching structure that enters the lobule (blue in b), is characterized as an arteriole for two reasons. Firstly, it is paired with a tubular airway seen in (a) in its most proximal portion as a lucent tubule, and subsequently interpolated in light blue in b. Secondly, it branches in the center of the lobule. It is distinct from the border forming interlobular septum which surrounds it. A second relatively large vessel colored in red receives a branch from the interlobular septum and by virtue of its size and position it has to be a pulmonary venule. We know that the lymphatic vessel accompanies the venule, and so the yellow lymphatic has been implied but not visualized. We also know that connective tissue surrounds these two structures. In this instance, the matrix of the lobule that consists of the alveoli is less dense than it should be and is surrounded by normal alveoli. Lucency implies air trapping and air trapping implies small airway disease. Thus this image tells us that the criminal in this case of disorder is the small airway. We now can focus on the small airways with a pathological differential diagnosis, and from there plan the treatment.
Courtesy of: Ashley Davidoff, M.D.
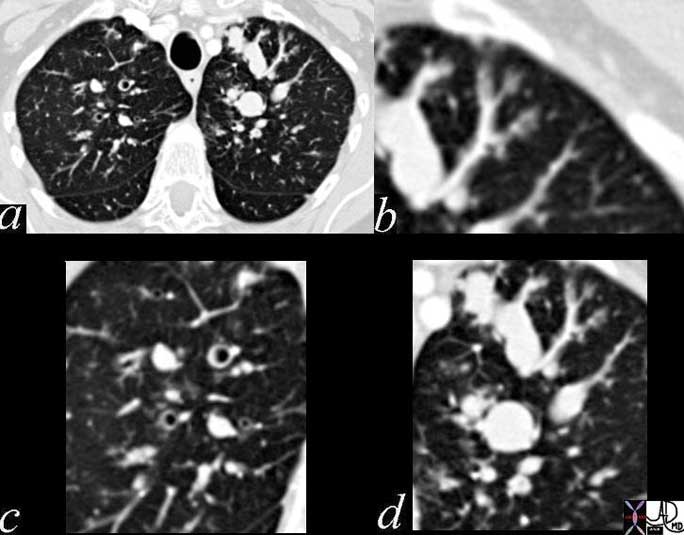
This case represents impacted bronchi and bronchioles with the organism aspergillus and the secondary inflammatory response to the fungus. The image in a, reveals a cross sectional view of the upper lung field, with tree in bud appearance seen in the most peripheral and anterior parts of the left upper lobe (b, d), partially filled bronchi in (c) and impacted distended bronchi in d.
Courtesy of: Ashley Davidoff, M.D.
Imaging of the Chest: The Alveoli
When the alveoli become diseased they fill to variable extent with fluid, whether it be pus, transudate, exudate, or cellular material, and radiologically present on CT with a pattern called ground glass when they are only half filled, or an alveolar pattern when they are fully filled. In the ground glass pattern there is a haze over the parenchyma, and we are still able to see vessels running through the density. In the alveolar consolidation the vessels cannot be seen since they have the same density as the alveoli, and we start to see air bronchograms.
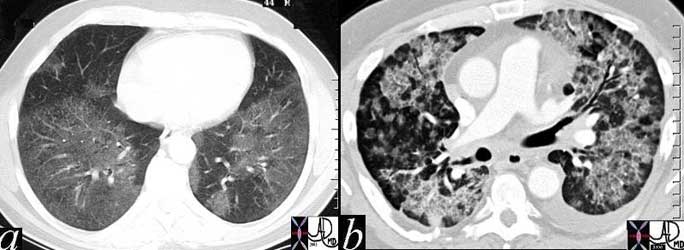
Image a, shows a ground glass pattern. Note we can see right through the density and the vessels are visualized in their entirety as they pass through the haze of the ground glass. In image b, we have frank alveolar change with the vessels becoming lost in the fog and the airways become more apparent exemplified by an air bronchogram in the anterior part of the left lung.
Courtesy of: Ashley Davidoff, M.D.
Conclusion
The following module presents the anatomic detail of the lung in great depth so that the finer points of diagnosis, particularly related to imaging and imaging techniques, can be elaborated. We have taken you through the anatomy of the bronchi on their journey to the histological level of the lung. Understanding the anatomy and histology is an essential step needed to understand lung imaging particularly in the world of high resolution CT. Imaging the chest is central to the management of the ICU patient where day-to-day life sustaining decisions are based on the CXR. Accurate placement of tubes and hardware is essential to survival. It is therefore important that meticulous care is taken to perform these studies in optimal fashion with optimal technique.
The innate contrast between the blackness of air and the whiteness of the soft tissues of the lungs gives us an unique opportunity to evaluate microscopic structures of the lungs, down to the thickening of sub-millimeter interstitial tissues. Prudent use of contrast and high resolution imaging has brought important understanding of lung disease. The consistent diagnosis of pulmonary embolism eluded us for years, and we now have a tool that has high sensitivity and specificty for this life threatening disease. PET scanning has added a functional tool that is essential in the management of the oncological patient, and has given us a non invasive method that has improved the diagnosis and hence the management of our patients.