- What is it:
- Cryptogenic Organizing Pneumonia (COP) is a
- non-infectious
- inflammatory lung disease
- characterized by:
- granulation tissue plugs in the
- bronchioles
- alveolar ducts and
- alveoli
- granulation tissue plugs in the
- Associated with
- chronic inflammation in the
- Surrounding interstitium.
- chronic inflammation in the
- It is classified as an
- idiopathic condition but
- can resemble
- infectious pneumonia
- clinically and on
- imaging
- Cryptogenic Organizing Pneumonia (COP) is a
- Etymology:
- Cryptogenic means of unknown origin.
- Derived from the Greek word organizein (to arrange), referring to the fibroblastic granulation tissue organizing in the airways.
- AKA:
- Idiopathic Bronchiolitis Obliterans Organizing Pneumonia (BOOP).
- What causes it:
- The exact cause is
- unknown, but it is considered
- idiopathic.
- Pathophysiologically, COP involves:
- Chronic inflammation
- Fibroblast proliferation,
- Intra-alveolar fibrosis
- .
- Chronic inflammation
- The exact cause is
- What is the result:
- Impaired gas exchange and respiratory symptoms due to:
- Although there is granulation tissue in the airways small airway obstruction is not a hallmark of COP,
- If untreated, it may progress to:
- Chronic respiratory dysfunction, or
- Rarely, pulmonary fibrosis.
- Impaired gas exchange and respiratory symptoms due to:
- How is it diagnosed:
- Clinical findings:
- Gradual onset of symptoms over weeks to months.
- Symptoms include:
- Persistent dry cough,
- Dyspnea,
- Fever, and
- Fatigue.
- Imaging studies:
- Applied Anatomy Diverse Findings
- Parts: Respiratory bronchioles, alveolar ducts, alveoli.
- Size: Lesions often span multiple secondary pulmonary lobules.
- Shape: Rounded, nodular, linear, or perilobular.
- Position: Can involve any lung region without lobar preference.
- Character:
- Ground-glass opacities
- Reticulations
- Consolidation,
- Atoll sign (reverse halo sign):
- Central ground-glass opacity surrounded by a
- ring of consolidation,
- historically considered characteristic but not pathognomonic.
- Nodules
- Masses
- Despite involvement of bronchioles mosaic attenuation and air trapping is not a common feature
- Time: Subacute onset over weeks to months.
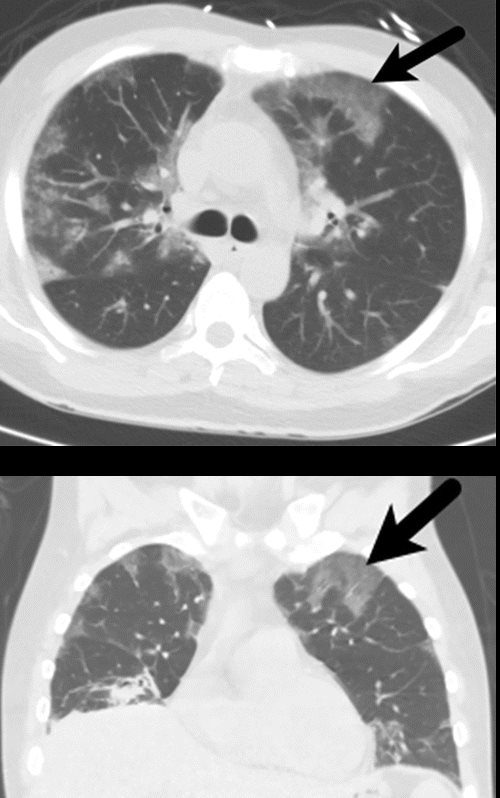
- Chest X-ray:
- Patchy, peripheral consolidations, often mimicking infectious pneumonia.
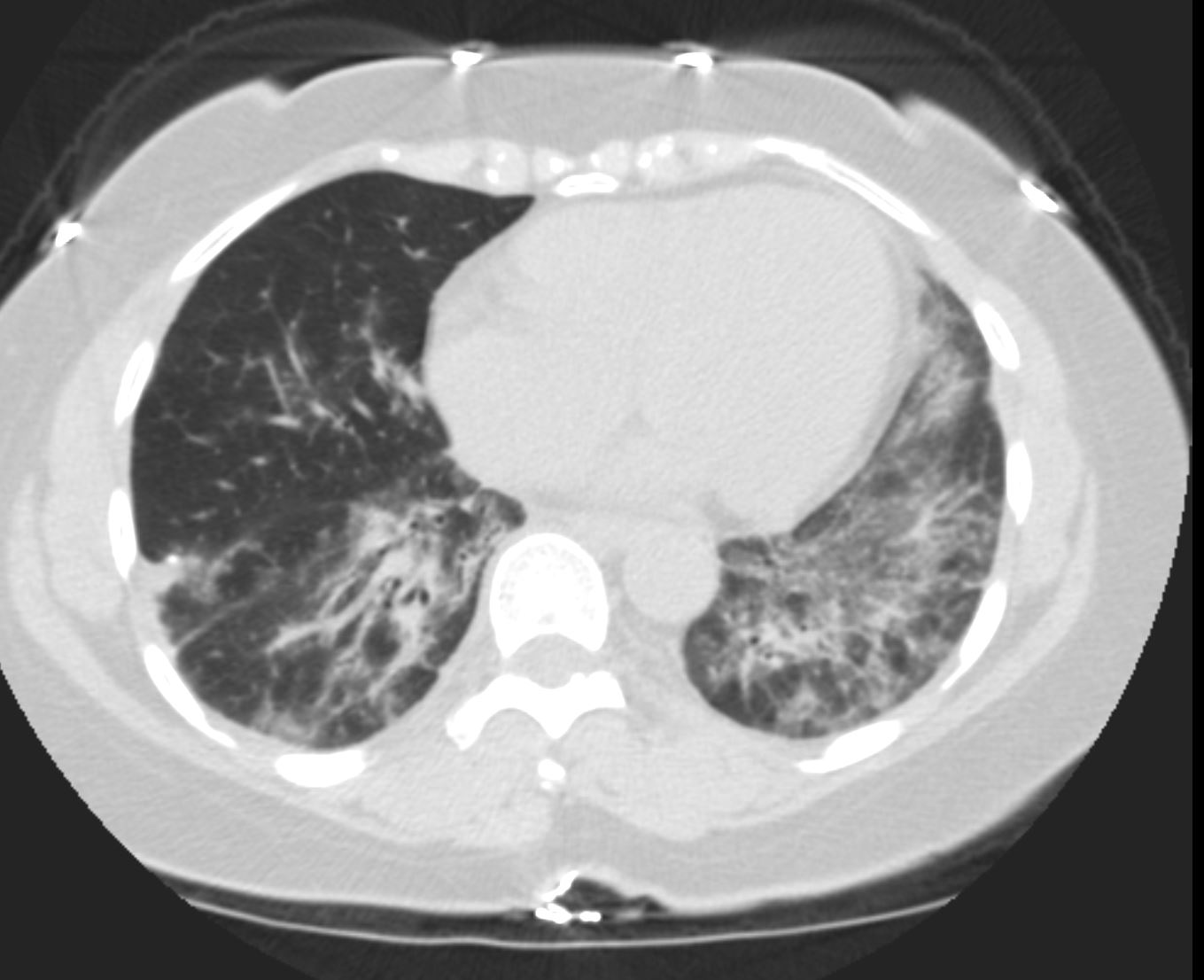
- Chest CT:
- Parts:
- Patchy airspace consolidations and
- nodules
- GGO’s.
- Size: Ranges from
- diffuse lung involvement
- segmental lesions to .
- focal subsegmental
- Position:
- Peripheral and basal lung field
- but can involve upper lung fields
- subpleural or
- peribronchial distribution,
- .
- Character:
- consolidations
- ground-glass opacities,
- nodular densities which can uncommonly cavitate
- interstitial changes.
- Atoll sign (reverse halo sign):
- Central ground-glass opacity surrounded by a
- ring of consolidation,
- historically considered characteristic but not pathognomonic.
- Time: Persistent over weeks but responsive to corticosteroid treatment.
- Parts:
- Applied Anatomy Diverse Findings
- Pulmonary Function Test (PFT):
- COP typically shows a restrictive pattern (reduced total lung capacity and vital capacity).
- Obstructive patterns, such as reduced forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity (FVC) ratio, are less common but may be seen in cases with significant small airway involvement.
- COP typically shows a restrictive pattern (reduced total lung capacity and vital capacity).
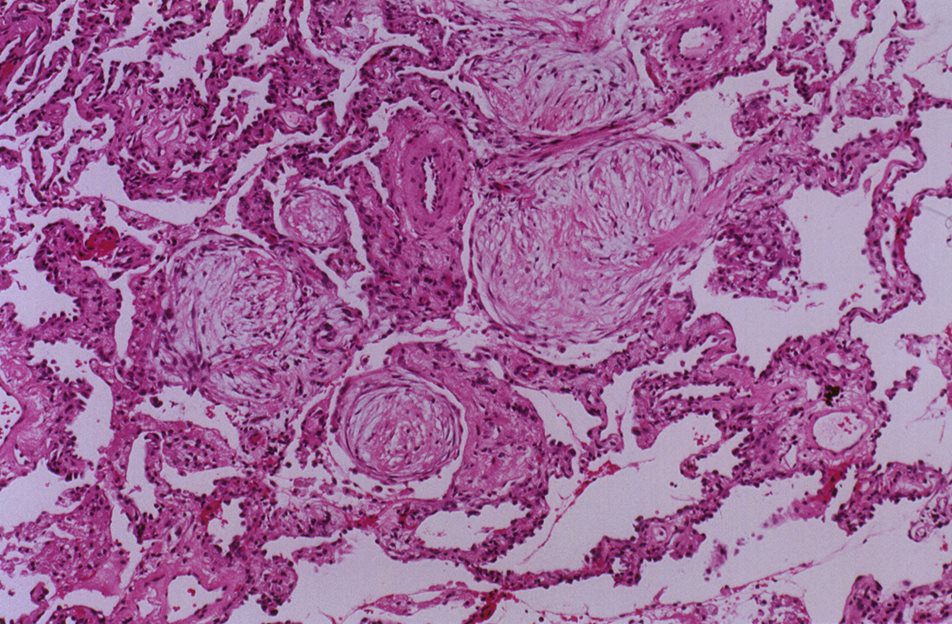
- Histopathology (gold standard):
- Fibroblastic plugs in
- bronchioles alveoli and (Masson bodies).
- Interstitial mononuclear inflammation and mild fibrosis.
- Fibroblastic plugs in
- Exclusion of other causes:
- Rule out infectious pneumonia, malignancy, and systemic diseases.
- Clinical findings:
- How is it treated:
- First-line therapy:
- Corticosteroids (e.g., prednisone): Rapid improvement in symptoms and imaging findings in most cases.
- Second-line options (for refractory cases):
- Immunosuppressive agents (e.g., azathioprine, cyclophosphamide).
- Supportive care:
- Oxygen therapy for hypoxemia.
- Pulmonary rehabilitation for chronic cases.
- Monitoring:
- Serial imaging to assess response to treatment and detect relapse.
- First-line therapy:
- Radiological implications:
- COP mimics infectious pneumonia on imaging but lacks microbiological evidence of infection.
- Serial imaging shows rapid improvement with corticosteroid therapy.
- Persistent or recurrent opacities may indicate incomplete response or relapse.
- Key points and pearls:
- Not infectious: Clinical history and microbiology are critical to differentiate COP from pneumonia.
- Imaging Has Diverse findings:
-
- OP can appear as
- bronchial wall thickening
ground-glass opacities - patchy consolidations,
- nodules,
- masses , or combinations thereof.
- Atoll sign (reverse halo sign): Once thought to be pathognomonic, it is now known to occur in other conditions (e.g., invasive fungal infections, vasculitis).
- Arcade like Sign
- Perilobular pattern
- Can present as focal masses, raising suspicion for malignancy.
- bronchial wall thickening
- OP can appear as
-
- Relapse is common: Close follow-up is necessary, as up to 50% of patients may experience recurrence.
- Early treatment with corticosteroids prevents progression to fibrosis.
- Exclude secondary organizing pneumonia (e.g., associated with connective tissue disease, drugs, or malignancy).
- Variety of Imaging Findings:
- COP can manifest as patchy consolidations, ground-glass opacities, nodules, masses, reticular opacities, and perilobular patterns.
- These findings can be focal, multifocal, or diffuse, with no strict lobe predilection.
- Specific signs like the Atoll sign (reverse halo sign) or the arcade-like sign are characteristic but not pathognomonic, as they are also seen in other conditions.
- Overlap with Other Diseases:
- Infectious pneumonia, particularly viral or fungal infections.
- Other interstitial lung diseases (e.g., NSIP, UIP).
- Neoplastic conditions like lung masses and lymphangitic carcinomatosis.
- Drug-induced lung injury or autoimmune-related organizing pneumonia.
- Mimics of Infectious Pneumonia:
- COP often clinically and radiologically resembles bacterial or atypical infections, especially when presenting as patchy consolidations or nodules.
- Dynamic Nature:
- Lesions may change with corticosteroid therapy, further complicating the diagnosis if there is partial resolution.
Diagnostic Challenges:
- Non-specific Findings: The common imaging findings are not exclusive to COP, requiring clinical, imaging, and sometimes histopathological correlation.
- Need for Biopsy: In cases where the imaging findings are ambiguous, a lung biopsy is often required to confirm the diagnosis.
Takeaway:
COP’s protean nature on imaging reinforces the importance of a multidisciplinary approach, combining clinical history, laboratory results, and imaging findings to reach a diagnosis.
Buzz The COPS are Everywhere and can Disguise as almost anything
- Structural
- peribronchial consolidation
- Interstitial
- alveoli
- bronchioles
- COPS All Lung Zones COPS can go anywhere
- bronchovascular
- peripheral
- Interstitial
- Path
- interstitial inflammation
- globs of tissue Masson bodies in in distal airspaces and bronchioles
- Imaging CT
- Ground Glass
- Patchy Consolidation
- Atoll Inverted Halo
- Nodules which can cavitate
“When an underlying cause is unknown it is classified as cryptogenic organizing pneumonia (COP; also referred to as primary organizing pneumonia) whereas if a cause is known it is then termed a secondary organizing pneumonia.”
Pathology – “characterized by granulation tissue polyps within alveolar ducts and alveoli and with chronic inflammation involving the adjacent lung parenchyma. ”
Most cases are cryptogenic
- Small Airway Disease – Organizing Pneumonia – Terminal Bronchiole
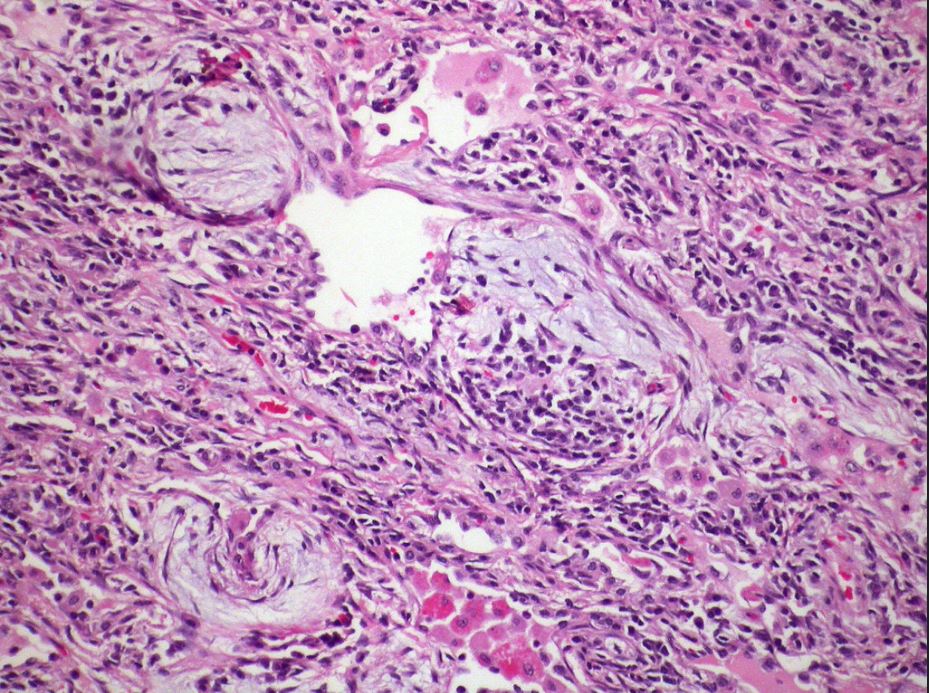
Hypersensitivity pneumonitis-organizing pneumonia Case 129
Areas of organizing pneumonia are frequently seen in hypersensitivity pneumonitis.
Courtesy Dr Yale Rosen- cryptogenic or secondary organizing pneumonia (OP),
-

Histopathology of organizing pneumonia (or BOOP), characterized by intraluminal plugs of proliferating fibroblasts that fill distal airways and peribonchiolar air spaces
Ryu, J Pulmonary Medicine Bronchiolitis Pulmonology Advisor
COP Pattern
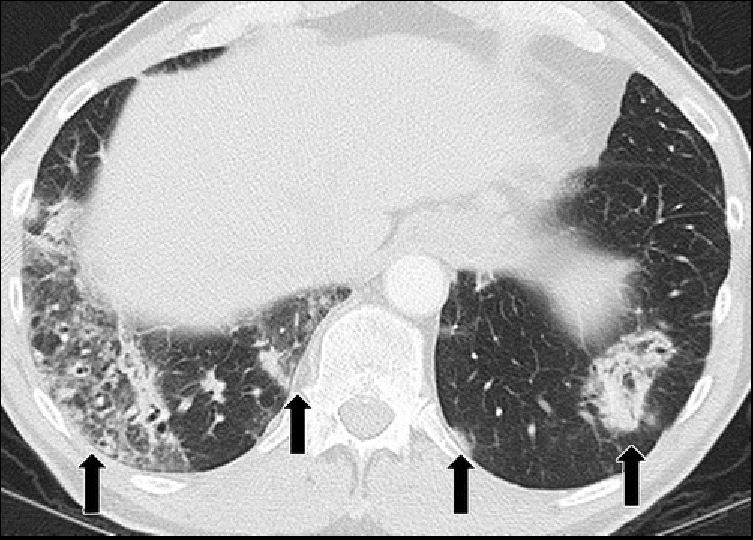
PD-1 inhibitor pneumonitis: COP pattern in a 69-year-old man with advanced NSCLC who was treated with nivolumab. At 6 months of therapy, the patient presented with increased shortness of breath and cough, without fever. Axial chest CT image shows multifocal areas of consolidation and GGOs in a predominantly peripheral and basilar distribution (arrows), representing a COP pattern of PD-1 inhibitor–related pneumonitis. Bronchial dilatation was noted within the areas of consolidation. Nivolumab was withheld, and the patient was treated with corticosteroids, with subsequent improvement.
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care Radio Graphics Vol. 37, No. 5
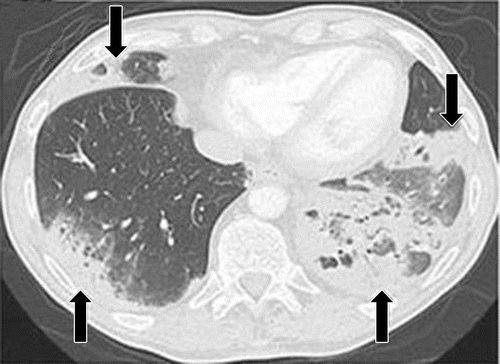
a
PD-1 inhibitor pneumonitis flare in a 72-year-old man with stage IV squamous NSCLC who was treated with nivolumab and presented with progressive dyspnea with cough and wheezing but no fever. (a) Axial chest CT image at 15 weeks of therapy demonstrates multifocal areas of GGOs, reticular opacities, and consolidation (arrows) involving all lobes, as well as centrilobular nodularity and traction bronchiectasis in a predominantly peripheral distribution. The overall features demonstrate a COP pattern. The patient was treated with prednisone for pneumonitis.
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care Radio Graphics Vol. 37, No. 5
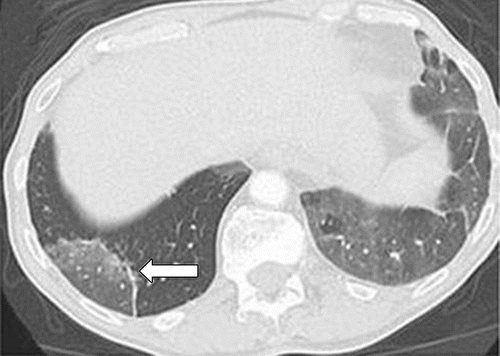
b
Axial follow-up CT image after 4 weeks of prednisone therapy shows a significant decrease in the findings, with residual GGOs. Note the “reversed halo” sign manifesting as a central GGO surrounded by a crecent-shaped dense airspace consolidation (arrow), a finding that has been reported as a radiologic manifestation of COP. (c) Axial CT image obtained 4 weeks after the completion of prednisone therapy shows the development of a bilateral dense consolidation with GGOs and reticular opacities (arrows) in peripheral and multifocal distributions, again demonstrating a COP pattern as noted during the first episode of PD-1 inhibitor pneumonitis. Given the similarity of the radiologic and clinical manifestations to those of the first episode, the patient restarted prednisone for treatment of pneumonitis flare. Follow-up chest CT images obtained 2 weeks after starting the second course of prednisone therapy (not shown) demonstrated a decrease in the findings, indicative of improving pneumonitis in response to corticosteroid therapy. (Figure 7 reprinted from reference 21.)
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care Radio Graphics Vol. 37, No. 5
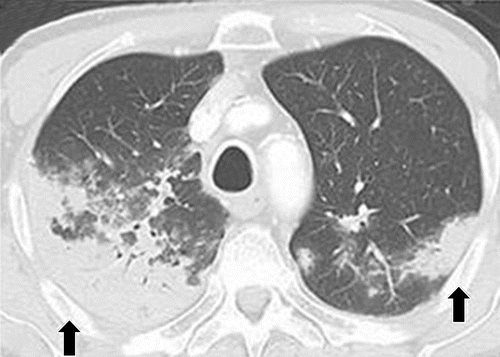
a
PD-1 inhibitor pneumonitis flare in a 72-year-old man with stage IV squamous NSCLC who was treated with nivolumab and presented with progressive dyspnea with cough and wheezing but no fever. (a) Axial chest CT image at 15 weeks of therapy demonstrates multifocal areas of GGOs, reticular opacities, and consolidation (arrows) involving all lobes, as well as centrilobular nodularity and traction bronchiectasis in a predominantly peripheral distribution. The overall features demonstrate a COP pattern. The patient was treated with prednisone for pneumonitis.
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care Radio Graphics Vol. 37, No. 5
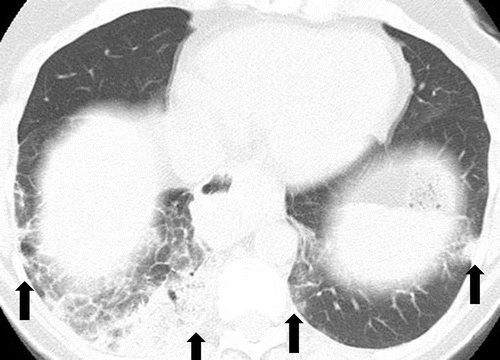
Pneumonitis in a 66-year-old woman with Waldenström macroglobulinemia treated with mTOR inhibitor therapy. Axial CT image at 6 months of therapy shows consolidation, GGOs, and reticular opacities (arrows) that represent a COP pattern.
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care Radio Graphics Vol. 37, No. 5

ALK inhibitor–related pneumonitis in a 55-year-old woman with ALK-positive stage IV adenocarcinoma of the lung. The patient experienced disease progression while taking a first-generation ALK inhibitor (crizotinib) and was then treated with a second-generation ALK inhibitor (ceritinib). She presented with an increasing dry cough and dyspnea at 7 months of ceritinib therapy. (a, b) Axial (a) and coronal (b) CT images show biapical consolidation and GGOs in both upper lobes (arrows) in a striking peripheral distribution, indicative of a COP pattern.
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care Radio Graphics Vol. 37, No. 5
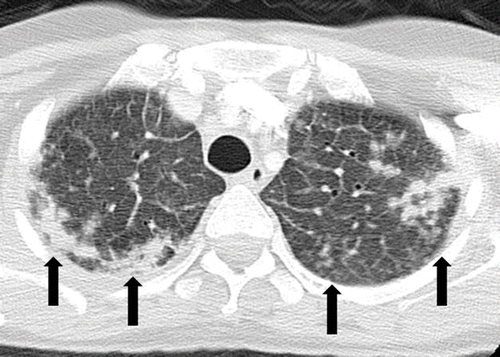
ALK inhibitor–related pneumonitis in a 55-year-old woman with ALK-positive stage IV adenocarcinoma of the lung. The patient experienced disease progression while taking a first-generation ALK inhibitor (crizotinib) and was then treated with a second-generation ALK inhibitor (ceritinib). She presented with an increasing dry cough and dyspnea at 7 months of ceritinib therapy. (a, b) Axial (a) and coronal (b) CT images show biapical consolidation and GGOs in both upper lobes (arrows) in a striking peripheral distribution, indicative of a COP pattern.
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care Radio Graphics Vol. 37, No. 5
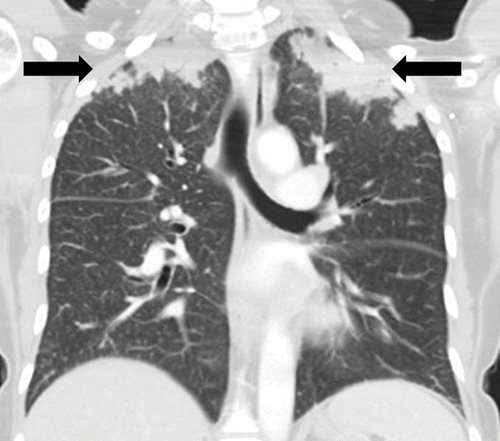
ALK inhibitor–related pneumonitis in a 55-year-old woman with ALK-positive stage IV adenocarcinoma of the lung. The patient experienced disease progression while taking a first-generation ALK inhibitor (crizotinib) and was then treated with a second-generation ALK inhibitor (ceritinib). She presented with an increasing dry cough and dyspnea at 7 months of ceritinib therapy. (a, b) Axial (a) and coronal (b) CT images show biapical consolidation and GGOs in both upper lobes (arrows) in a striking peripheral distribution, indicative of a COP pattern.
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care Radio Graphics Vol. 37, No. 5
c
(c, d) Photomicrographs from transbronchial lung biopsy specimen show organizing interstitial pneumonia characterized by alveolar interstitial widening by lymphocytic infiltrates, increased extracellular matrix material, reactive pneumocyte hyperplasia, scattered eosinophils (arrow in d), and numerous airspace foamy macrophages. There was no evidence of tumor in the biopsy specimen. (Hematoxylin-eosin stain; original magnification, ×200 in c, ×400 in d.)
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care RadioGraphicsVol. 37, No. 5
d
(c, d) Photomicrographs from transbronchial lung biopsy specimen show organizing interstitial pneumonia characterized by alveolar interstitial widening by lymphocytic infiltrates, increased extracellular matrix material, reactive pneumocyte hyperplasia, scattered eosinophils (arrow in d), and numerous airspace foamy macrophages. There was no evidence of tumor in the biopsy specimen. (Hematoxylin-eosin stain; original magnification, ×200 in c, ×400 in d.)
Nishino, M et al Thoracic Complications of Precision Cancer Therapies: A Practical Guide for Radiologists in the New Era of Cancer Care RadioGraphicsVol. 37, No. 5
-

Organizing pneumonia. (a) Axial and (b) coronal images in lung windows in a 43-year-old man with a history of follicular lymphoma demonstrate predominantly peripheral ground-glass opacities (arrow). The patient had undergone chemotherapy and was admitted for respiratory failure. Lung biopsy yielded organizing pneumonia. The patient did well on steroids and was discharged.
Parekh, M et al Review of the Chest CT Differential Diagnosis of Ground-Glass Opacities in the COVID Era Radiology Vol. 297, No. 3 July 2020
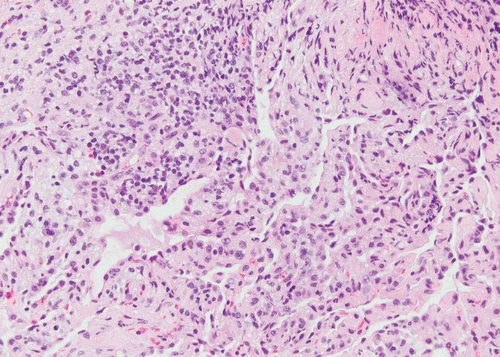
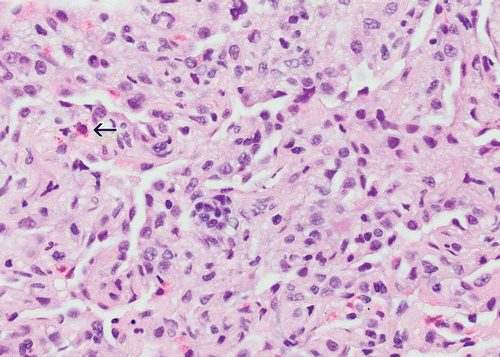
Case of a patient in remission from adult T-cell lymphoma/leukemia, now with multiple PET, AVID lung nodules Pathology showed Acute organizing pneumonitis which could be part of any condition in the lung such as an infection. CT showed cavitating nodules and solid nodules which were PET AVID The conclusion was that even though the pathology showed organizing pneumonitis, it could still be a response to the infection
Cavitating and solid nodules PATH Acute organizing pneumonitis
Ashley Davidoff thecommonvein.net
Ashley Davidoff thecommonvein.net
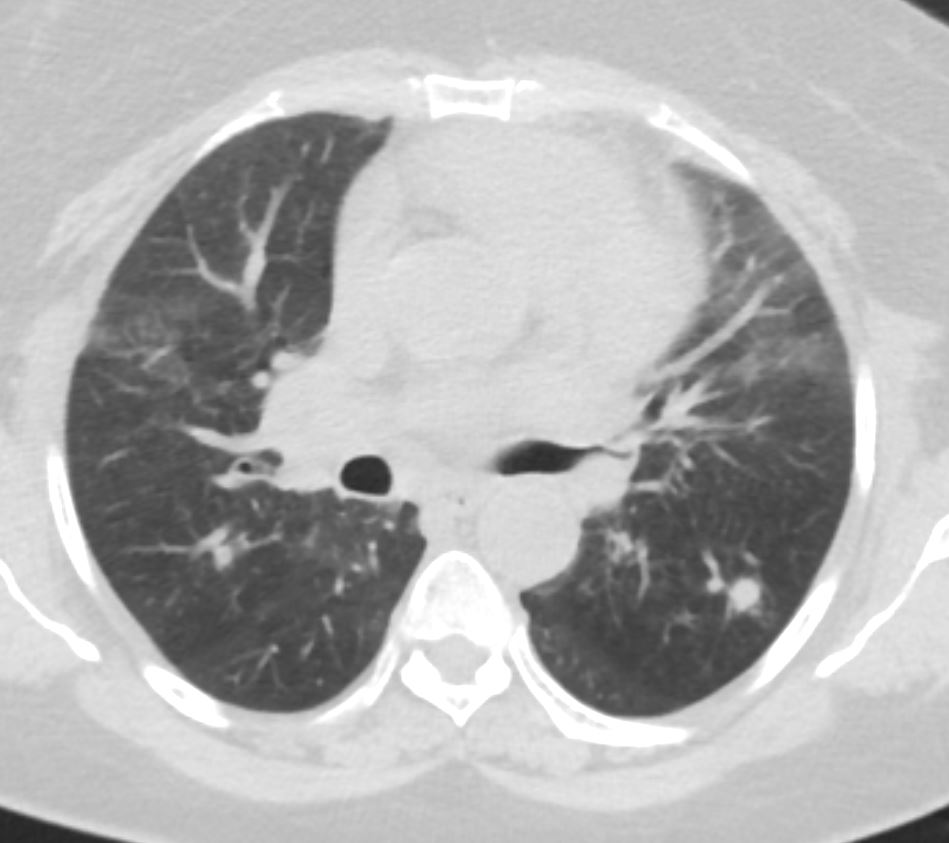
Ashley Davidoff thecommonvein.net
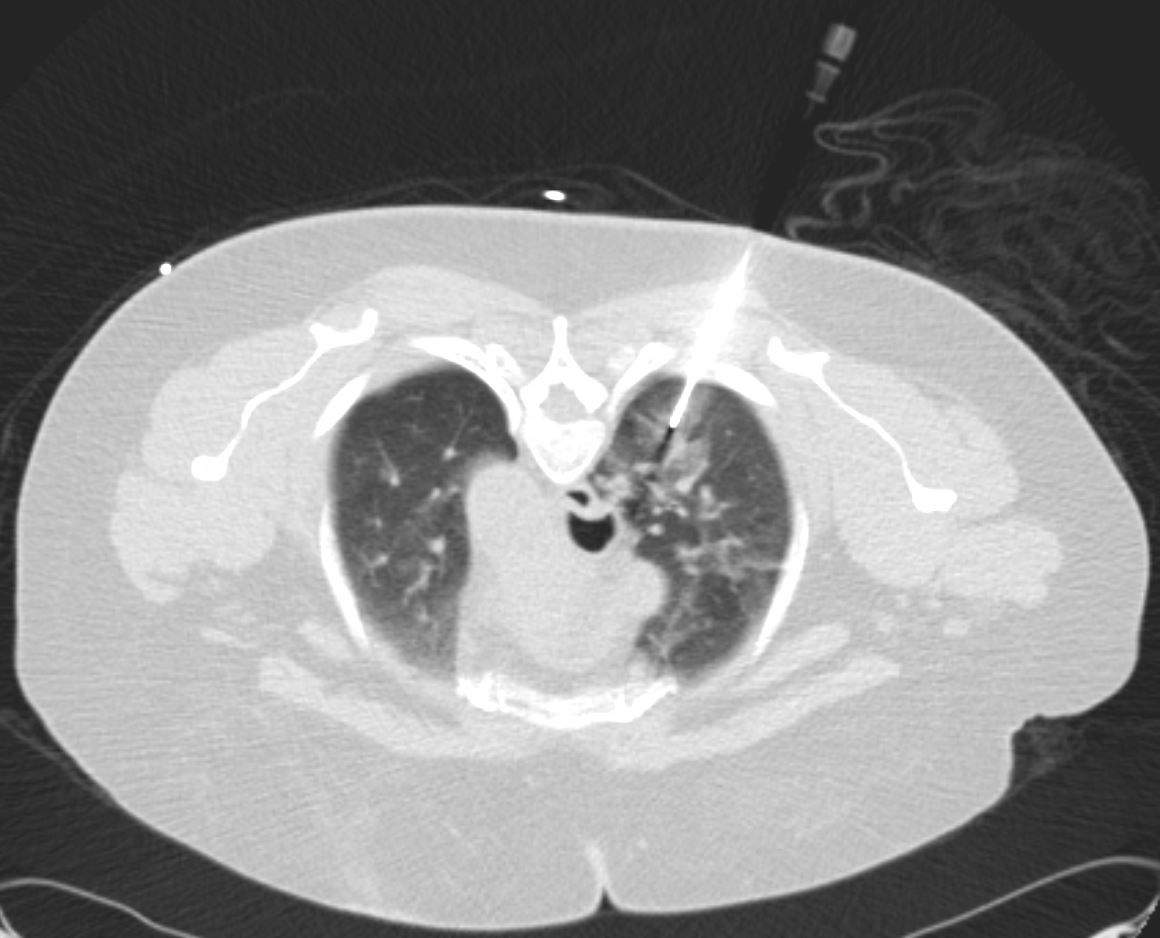
Ashley Davidoff thecommonvein.net
COP vs NSIP
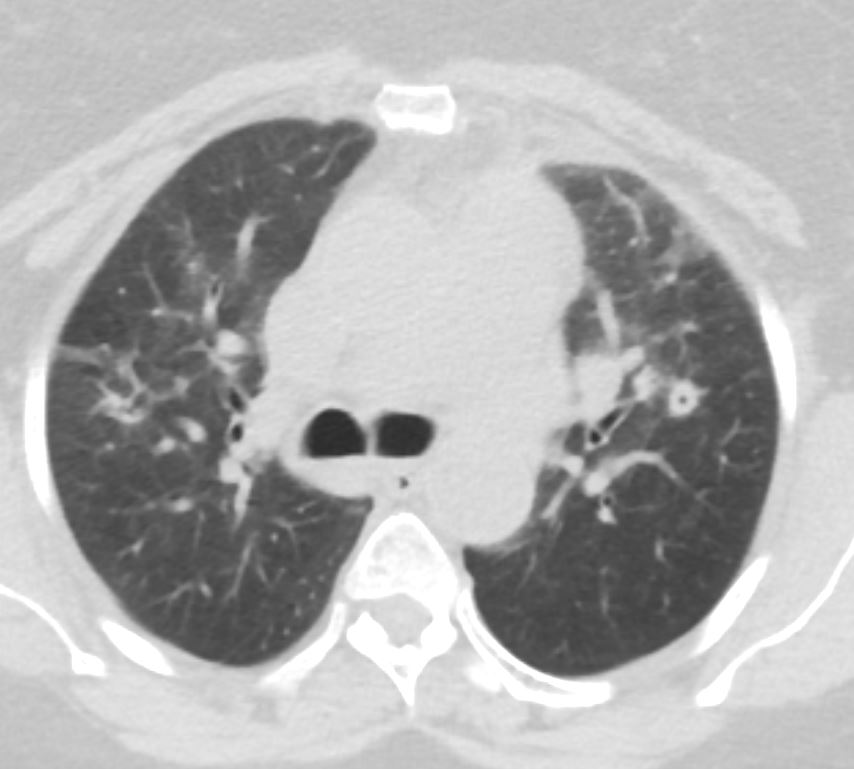
56 year old female presents with CT findings of basilar bronchovascular infiltrates, almost symmetrical, associated with mediastinal and axillary adenopathy.
Pathological report was complex but suggested a diagnosis of cryptogenic organizing pneumonia Ashley Davidoff MD TheCommonVein.net
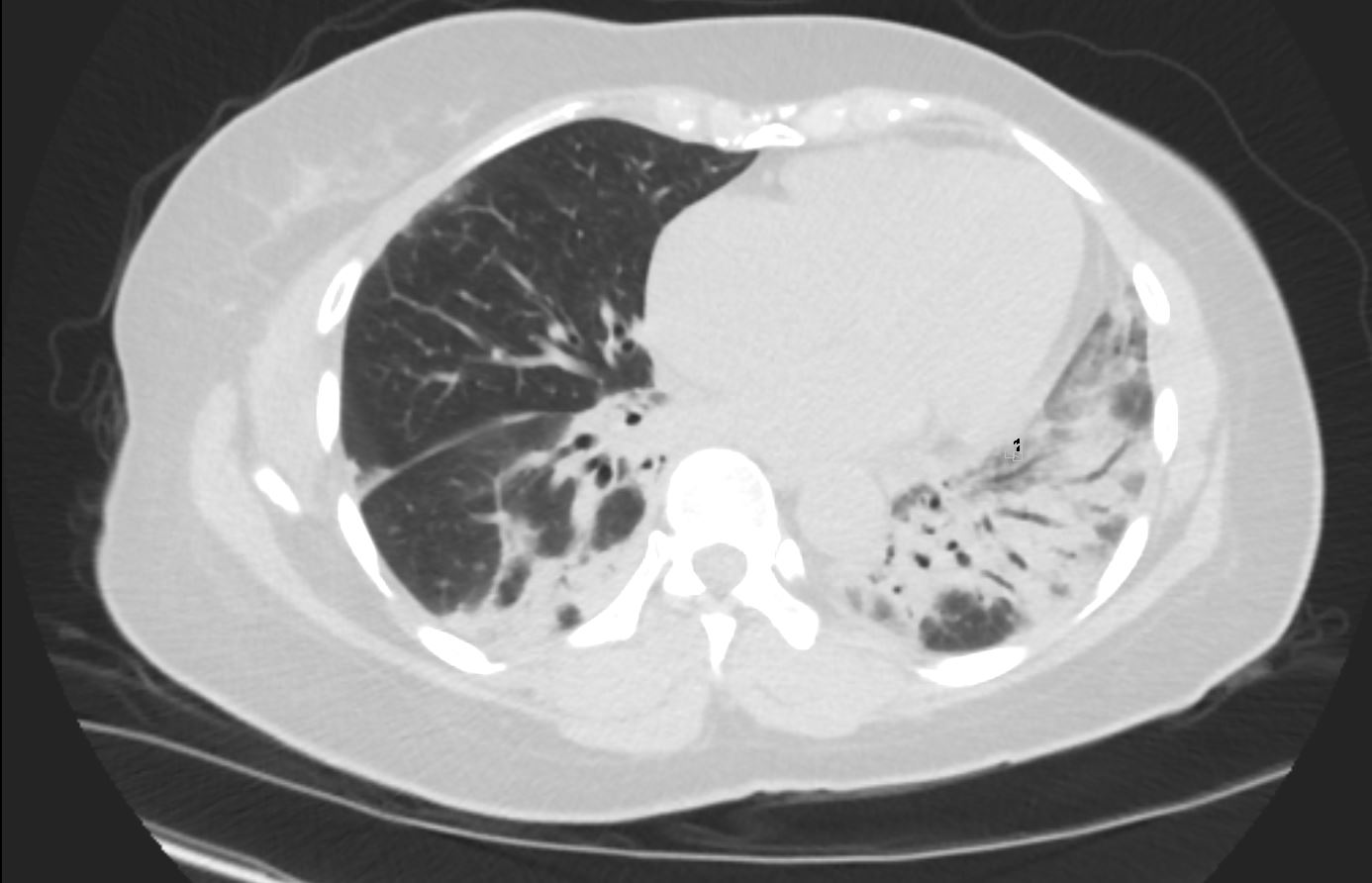
56 year old female presents with CT findings of basilar bronchovascular infiltrates, almost symmetrical, associated with mediastinal and axillary adenopathy.
Pathological report was complex but suggested a diagnosis of cryptogenic organizing pneumonia
Ashley Davidoff MD TheCommonVein.net
1 Year Later
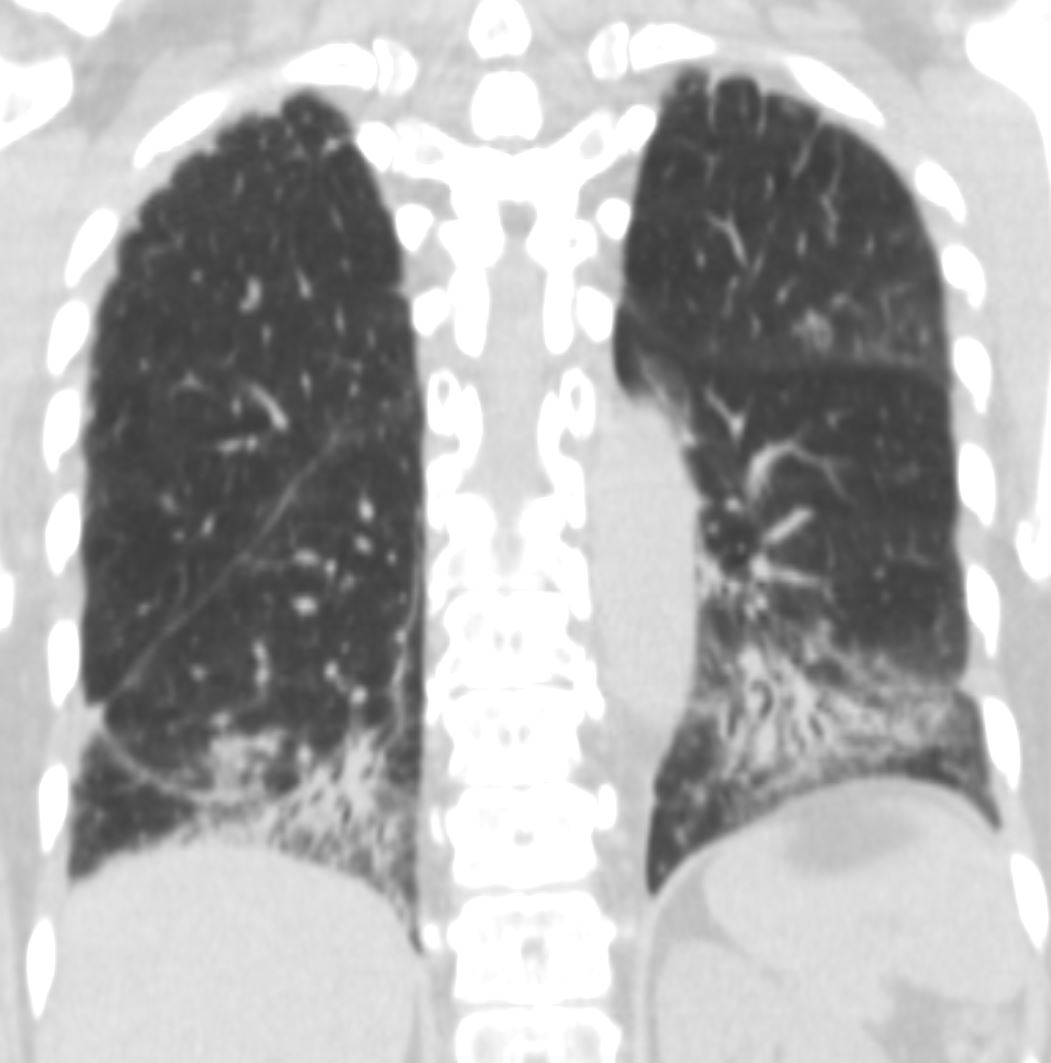
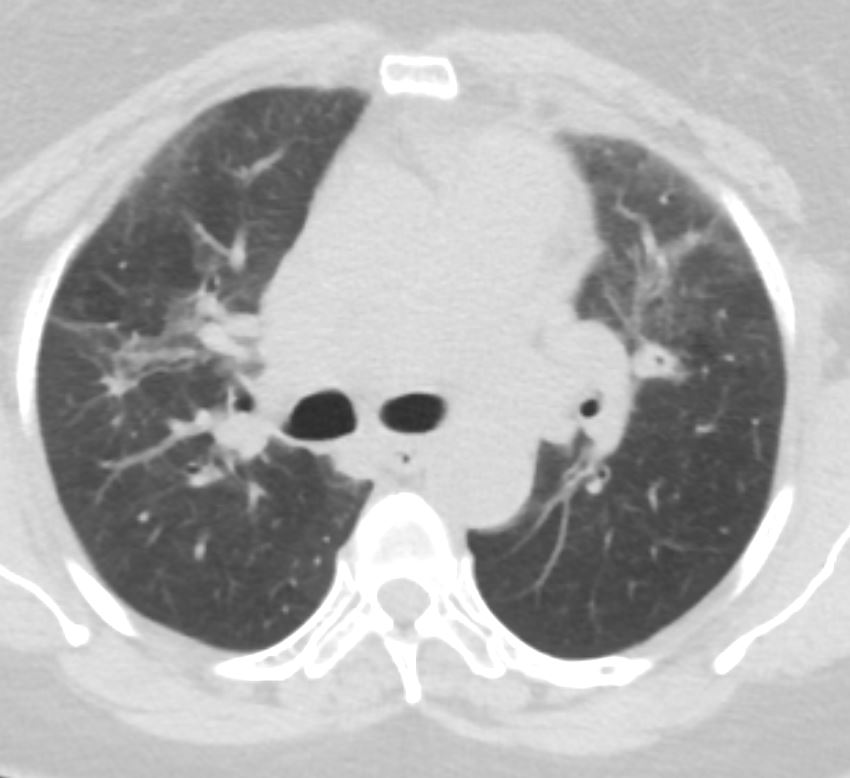
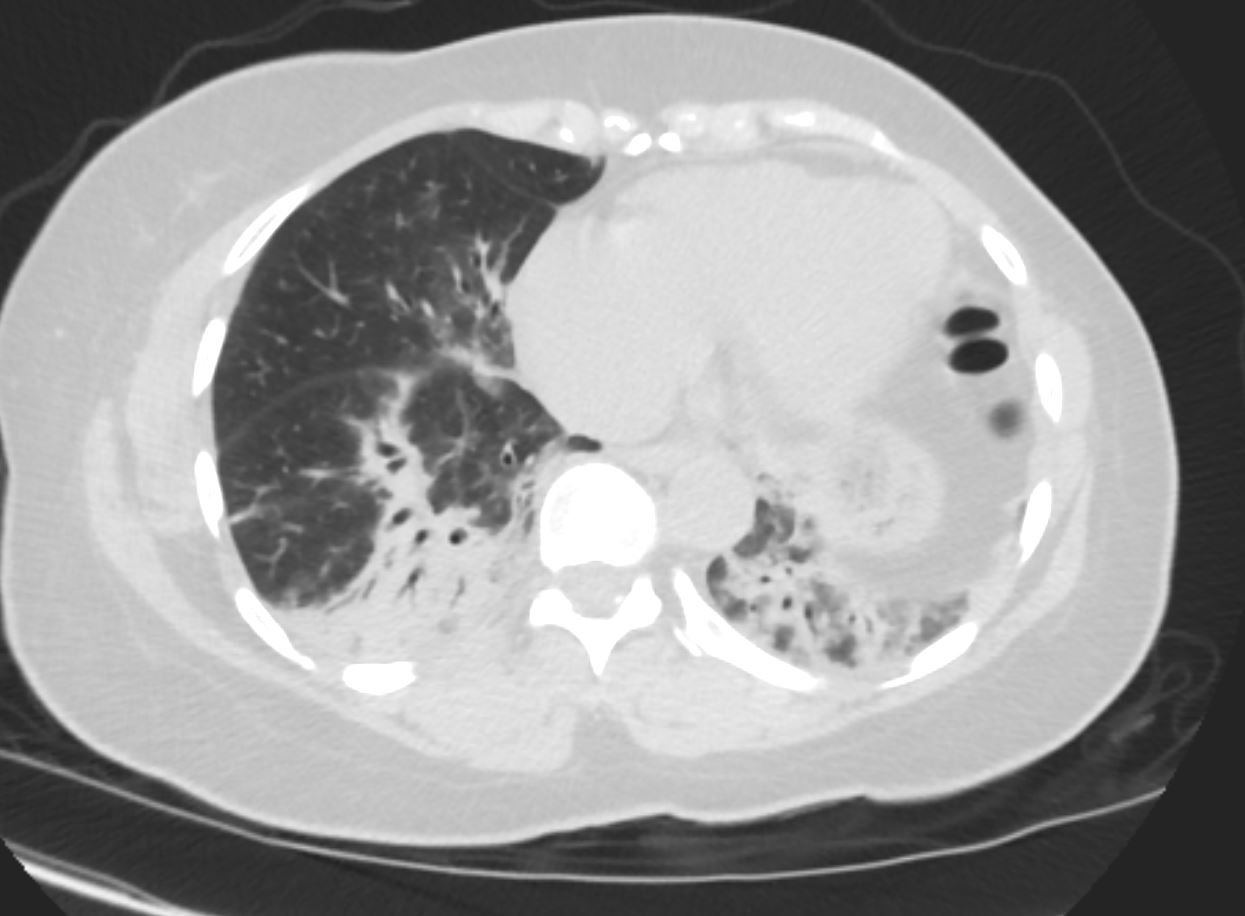
57 year old female presents 1 year later with similar CT findings of basilar bronchovascular infiltrates, almost symmetrical, associated with mediastinal and axillary adenopathy.
Pathological report was complex but suggested a diagnosis of cryptogenic organizing pneumonia
Ashley Davidoff MD
COP vs NSIP
57 year old female presents 1 year later with similar CT findings of basilar bronchovascular infiltrates, almost symmetrical, associated with mediastinal and axillary adenopathy.
Pathological report was complex but suggested a diagnosis of cryptogenic organizing pneumonia
Ashley Davidoff MD TheCommonVein.net
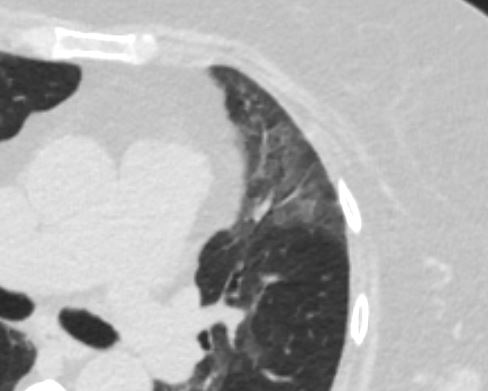
Pathology proven diagnosis
Ashley Davidoff MD TheCommonVein.net
References and Links
Tiralongo et al Cryptogenic Organizing Pneumonia: Evolution of Morphological Patterns Assessed by HRCT Diagnostics 2020, 10(5), 262;
- TCV
- Cases