- What is it:
- Desquamative Interstitial Pneumonia (DIP) is a
- rare,
- smoking-related interstitial lung disease (ILD) characterized by:
- The accumulation of
- smoker’s macrophages (pigmented macrophages) in the alveoli.
- Mild
- interstitial inflammation and
- fibrosis of the alveolar walls.
- The accumulation of
- Despite its name,
- “desquamation”
- does not involve epithelial cell shedding but
- refers to the presence of
- intra-alveolar macrophages.
- “desquamation”
- Desquamative Interstitial Pneumonia (DIP) is a
- Etymology:
- Derived from the Latin word desquamare (to scrape off or shed), which historically misrepresented the histological findings.
- AKA:
- Smoking-related interstitial pneumonia, Alveolar macrophage pneumonitis.
- What causes it:
- Primary cause:
- Cigarette smoking is the most common and strongly associated cause.
- Other potential causes (rare):
- Environmental exposures (e.g., dust, fumes).
- Certain medications (e.g., chemotherapy agents).
- Autoimmune diseases (rare).
- Primary cause:
- What is the result:
- Chronic inflammation of the alveoli leads to:
- Impaired gas exchange, and
- Progressive pulmonary fibrosis in some cases.
- If untreated or if smoking continues, it may progress to fibrotic ILD.
- Chronic inflammation of the alveoli leads to:
- How is it diagnosed:
- Clinical findings:
- Gradual onset of symptoms, typically in middle-aged smokers.
- Symptoms include:
- Progressive dyspnea,
- Chronic dry cough, and
- Fatigue.
- Imaging studies:
- Chest X-ray:
- Diffuse reticular or ground-glass opacities, more pronounced in the lower lobes.
- Chest CT:
- Parts: Patchy ground-glass opacities with subpleural and basal predominance.
- Size: Diffuse involvement of alveolar spaces, without discrete nodules.
- Shape: Homogeneous or patchy.
- Position: Predominantly lower lobes, peripheral or subpleural.
- Character:
- Absence of honeycombing (helps differentiate from UIP).
- Mild interstitial thickening may be seen.
- Time: Progressive without smoking cessation but reversible in early stages with treatment and lifestyle changes.
- Chest X-ray:
- Histopathology (gold standard):
- Alveolar spaces filled with macrophages containing pigmented material (“smoker’s macrophages”).
- Mild interstitial fibrosis and chronic inflammatory cell infiltrates.
- Exclusion of other diseases:
- Rule out other smoking-related ILDs (e.g., respiratory bronchiolitis-associated ILD [RB-ILD], pulmonary Langerhans cell histiocytosis).
- Clinical findings:
- How is it treated:
- Smoking cessation:
- The cornerstone of treatment. Most cases improve or stabilize if smoking is stopped.
- Corticosteroids (e.g., prednisone):
- Effective in reducing inflammation in symptomatic cases or progressive disease.
- Immunosuppressive therapy:
- May be considered in corticosteroid-refractory cases.
- Supportive care:
- Pulmonary rehabilitation and oxygen therapy for advanced disease.
- Monitoring:
- Serial pulmonary function tests (PFTs) and imaging to assess response and progression.
- Smoking cessation:
- Radiological implications:
- DIP findings are reversible with early smoking cessation and corticosteroid treatment.
- Serial imaging is crucial to monitor resolution or progression to fibrosis.
- Absence of honeycombing distinguishes DIP from UIP.
- Key points and pearls:
- Smoking-related ILD: DIP is almost exclusively found in smokers or individuals with significant smoking history.
- Ground-glass opacities: Lower lobe and peripheral distribution are hallmark features.
- Potential for reversibility: Early diagnosis and smoking cessation are critical to prevent progression.
- Differentiation: DIP must be distinguished from RB-ILD (which has more proximal airway involvement) and UIP (which shows honeycombing and more pronounced fibrosis).
- Histopathology clarity: Despite its name, “desquamation” refers to intra-alveolar macrophages, not epithelial cell shedding.
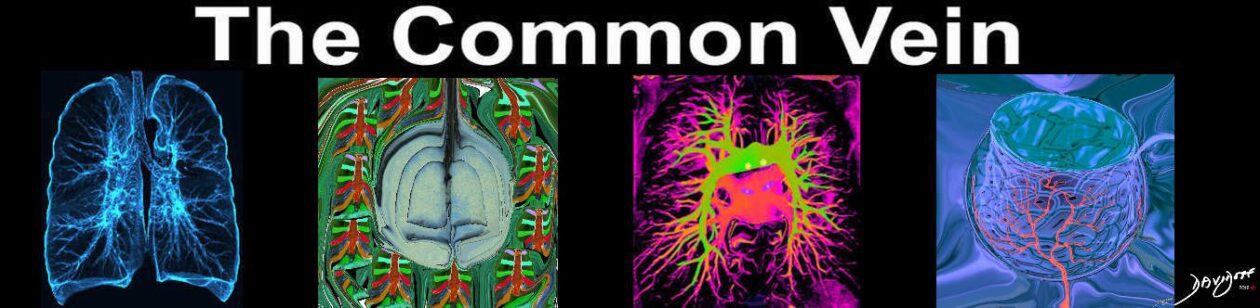
Lower Lobe distribution
Ashley Davidoff MD TheCommonvein.net lungs-0771
Predominantly Lower Lobes Peripheral
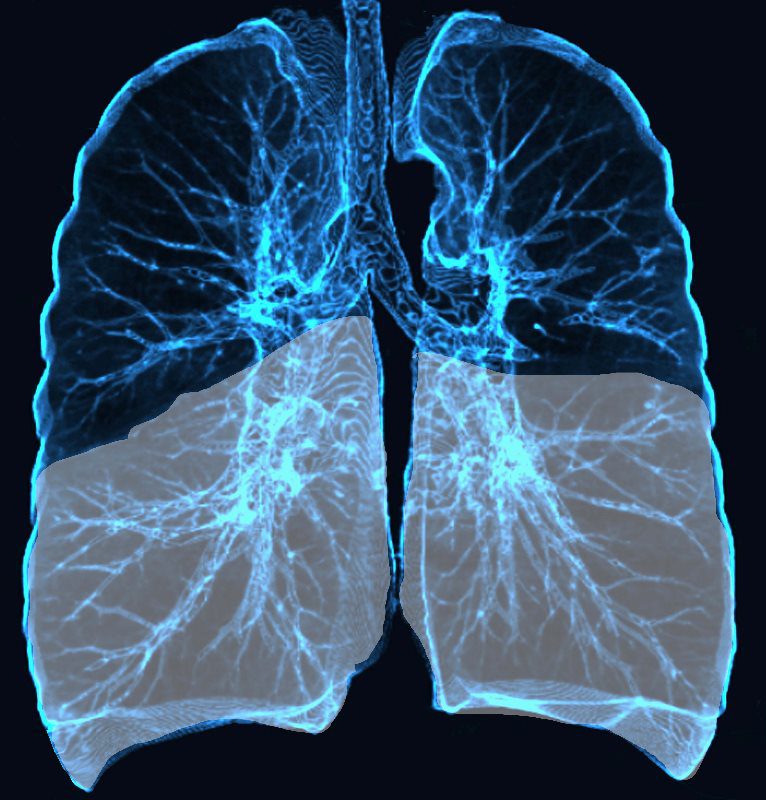
As the disease progresses the lower disease becomes more extensive and the disease progresses into the periphery of the upper lobes as well
Ashley Davidoff MD TheCommonvein.net lungs-0769c
aka alveolar macrophage pneumonia
a less angr brother to RB ILD
- Inflammatory
- pigmented intra-alveolar macrophages and giant sells in the alveioli
- Cause
- Mostly smokers
- 10-40% are non smokers
- inhalational toxic
- druga
- viral illness
- autoimmune diseases
- 10-40% are non smokers
- Mostly smokers
- results
- Structural
- Path
- alveoli
- architecture maintained
- macrophages
- uniform diffuse
- Interstitium
- mild chronic inflammation
- alveoli
- CXR
- usually normal
- CT
- widespread
- patchy ground glass
- diffuse less common
- lower lobes (70%)
- bilateral
- relatively symmetrical
- peripheral (60%)
- honeycomb can happen
- Path
- Functional
- LFT mild impairment
- Structural
Buzz
- DIP
- aberrances
- DIP imagine somebody dipping a rag of into cigarette smoke and dabbing it in the lower lung zones smoke
- aberrances
Lungs
- Desquamative –
- squams = misnomer
- infiltrates represent alveolar filling with pigmented macrophages. alveoli
interstitial mucosa/submucosa vessels
pneumonia = “inflammation of the lungs,”
desquamate – fill the alveoli
Small Airways
Inflammation
Secondary Lobule
Alveoli
Inhalation – Lower Lobes
Smokers but
- Desquamative Interstitial Pneumonia (DIP) is a rare form of idiopathic interstitial pneumonia (IIP).
- Cause
- Smokers
- also related to marijuana smoke inhalation,
- infections such as HIV,
- toxins, or
- occupational exposure (eg, to asbestos)
- Smokers
- Results in
- infiltrates represent alveolar filling with pigmented macrophages. alveoli
- Diagnosis
- Clinical
- Cougn Dyspnea
- DX difficult on
- clinical and radiological features alone
- requires a (surgical) lung biopsy
- Imaging
- bilateral ground-glass opacities with
- lower lobe predominance (92%).
- irregular reticulation,
- traction bronchiectasis and
- cysts.
- sometimes
- ground-glass opacities
- architectural distortion,
- traction bronchiectasis and
- honeycombing.
- Clinical
- Path
- pigmented macrophages within
- most of the distal airspace of the lung
- Histologically,
- DIP is similar to RB-ILD,
- DIP and RB-ILD are a spectrum
- differing in compartments involved
- DIP not bronchiolocentric.
- hyperplasia of the alveolar type II cells
- distribution pattern more homogeneous a
- mild peribronchial fibrosis
- DIP is similar to RB-ILD,
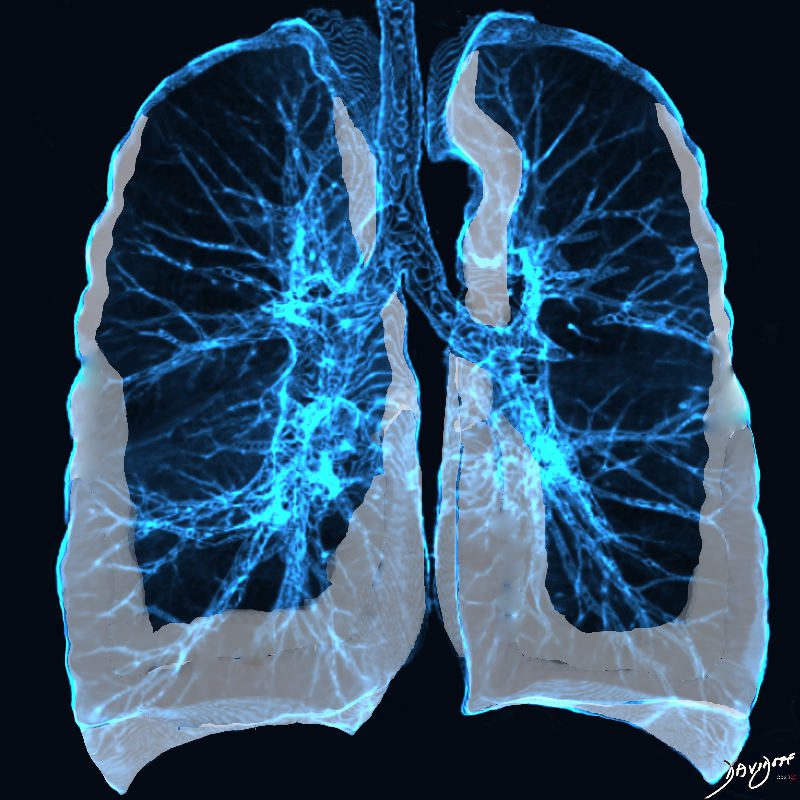
Anil K Smoking related ILD Radiographics

Noted are elevated numbers of macrophages within the alveoli of the lung. The alveolar macrophages have a characteristic light brown pigmentation and accumulate in the alveolar lumen and septa regions of the lower lobes of the lungs. The typical effects of the macrophage accumulation are inflammation and later fibrosis with stiffness of the lung
Courtesy Wiki
web lungs 436

Hellemons M etal Eur Respir Rev 2020; 29: 190181. – September 30, 2020
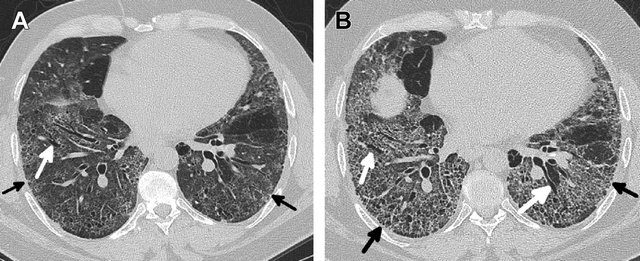
Kligerman S et al Clinical-Radiologic-Pathologic Correlation of Smoking-Related Diffuse Parenchymal Lung Disease 2016
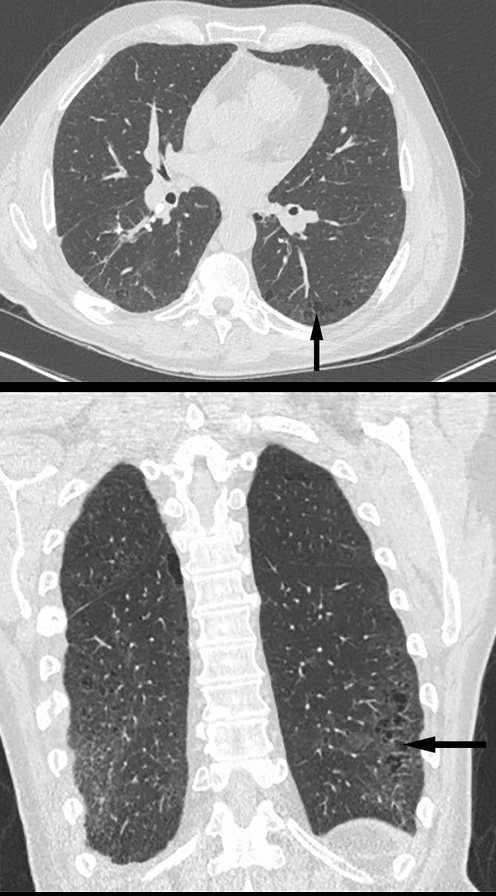
Parekh, M et al Review of the Chest CT Differential Diagnosis of Ground-Glass Opacities in the COVID Era Radiology Vol. 297, No. 3 July 2020
Radiology Buzz
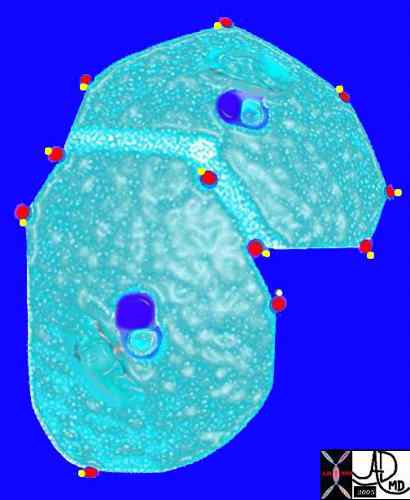
The arteriole and bronchiole lie in the center of the lobule.
Pulmonary venules (red) and lymphatics (yellow). lie in the periphery of the lobule
42440b03
Davidoff Art Courtesy Ashley Davidoff MD
References and Links
Attili, A.K etal Smoking-related Interstitial Lung Disease: Radiologic-Clinical-Pathologic Correlation RadioGraphics Vol. 28, No. 5
Gupta et al Diffuse Cystic Lung Disease: Part I American Journal of Respiratory and Critical Care Medicine 191(12) April 2015
-
TCV
- TCV
-
Videos
-
- Usual interstitial pneumonia (UIP)
- Nonspecific interstitial pneumonia (NSIP)
- Cryptogenic organizing pneumonia (COP)
- Desquamative interstitial pneumonia (DIP)
- Respiratory bronchiolitis-interstitial lung disease (RB-ILD)
- Acute interstitial pneumonia (AIP)
- Lymphoid interstitial pneumonia (LIP)
- Idiopathic pleuroparenchymal fibroelastosis (PPFE)