Etymology
Derived from Latin terms “septum” (partition) and “interlobular” (between lobules), referring to the connective tissue structures delineating secondary pulmonary lobules.
AKA and abbreviation
Interlobular septal thickening (IST).
What is it?
Interlobular septal thickening refers to the abnormal thickening of the connective tissue walls (septa) that separate adjacent secondary pulmonary lobules. It is a radiologic finding indicative of underlying diseases affecting the lung interstitium.
Characterized by
Thickened or irregular septa that become visible on imaging modalities, particularly CT and CXR.
Can appear:
- Smooth: Due to fluid accumulation (e.g., pulmonary edema).
- Nodular: Resulting from lymphatic or neoplastic infiltration.
- Irregular: Associated with fibrosis or chronic interstitial diseases.
Nodules along thickened septa are most commonly of soft tissue density but may occasionally be calcified, especially in granulomatous diseases such as sarcoidosis or silicosis.
Kerley B lines describe short, horizontal linear opacities caused by smooth thickening of interlobular septa, most prominent in the lung periphery.
Kerley Lines Overview
- Kerley A lines:
- Location: Radiate obliquely from the hila into the upper lobes.
- Length: Long (2–6 cm).
- Anatomy reflected: Distended lymphatic channels along bronchovascular bundles.
- Frequency: Rare, typically seen in pulmonary venous hypertension.
- Kerley B lines:
- Location: Appear at the lung bases, perpendicular to the pleural surface.
- Length: Short (1–2 cm).
- Anatomy reflected: Thickened interlobular septa due to fluid accumulation.
- Frequency: Not commonly seen but are the most frequent radiologic manifestation of interstitial thickening in heart failure.
- Kerley C lines:
- Location: Diffuse reticular pattern across the lung fields.
- Length: Short, intersecting lines without specific orientation.
- Anatomy reflected: Likely summation of Kerley B lines in central areas.
- Frequency: Rare and difficult to distinguish.
Crazy Paving Sign
The crazy paving sign is a radiologic pattern seen on CT, characterized by thickened interlobular septa superimposed on a background of ground-glass opacities, giving a mosaic or “cobblestone-like” appearance.
Causes of Crazy Paving:
- Infectious: Pneumocystis pneumonia, COVID-19-related acute respiratory distress syndrome.
- Inflammatory/Immune: Acute eosinophilic pneumonia, hypersensitivity pneumonitis.
- Metabolic: Pulmonary alveolar proteinosis (classic cause), lipoid pneumonia, amyloidosis.
- Hemorrhagic: Diffuse alveolar hemorrhage.
- Other: Fat embolism syndrome, drug-induced lung injury.
Caused by
- Most common cause: Pulmonary venous congestion (smooth thickening in left heart failure).
- Other causes include:
- Infection: Tuberculosis (calcified septa), Pneumocystis pneumonia.
- Inflammation/Immune: Sarcoidosis (nodular), acute eosinophilic pneumonia, hypersensitivity pneumonitis. alveolar proteinosis
- Neoplasm: Lymphangitic carcinomatosis (commonly nodular thickening).
- Mechanical Trauma: Rare, such as post-contusion changes.
- Metabolic: Pulmonary alveolar proteinosis, chronic renal failure (calcifications). alveolar proteinosis
- Circulatory: Pulmonary venous obstruction, pulmonary hemorrhage.
- Inherited/Congenital: Congenital pulmonary lymphangiectasia.
- Other: Drug toxicity, post-radiation fibrosis.
Resulting in:
Impaired gas exchange, interstitial lung pattern on imaging, and varied respiratory symptoms depending on the underlying cause.
Structural changes:
Thickening of interlobular septa due to:
- Fluid accumulation (e.g., edema, venous congestion).
- Lymphatic congestion.
- Cellular infiltration (e.g., inflammatory, neoplastic).
- Fibrosis or calcifications.
Pathophysiology:
- Smooth thickening: Increased hydrostatic pressure in pulmonary veins or lymphatic congestion.
- Nodular thickening: Cellular infiltration (e.g., sarcoidosis, carcinoma).
- Calcified thickening: Chronic inflammation or metabolic deposition processes.
- Crazy paving sign: Simultaneous alveolar and septal involvement due to mixed pathologies.
Pathology:
- Thickened septa contain lymphatic vessels, venules, and fibrous tissue.
- Changes include fluid, cellular infiltrate, or calcified deposits.
Diagnosis:
Based on clinical and radiologic correlation, often requiring histologic confirmation for nodular or calcified patterns.
Clinical:
Symptoms depend on the underlying cause and may include dyspnea, cough, or systemic signs like fever or weight loss.
Radiology:
- CXR:
- Findings:
- Kerley A, B, and C lines as described above.
- Associated Findings: Cardiomegaly, pleural effusions in venous congestion; nodules in sarcoidosis.
- Findings:
- CT:
- Parts: Interlobular septa separating secondary pulmonary lobules.
- Size: Thickened septa of variable thickness based on cause.
- Shape: Smooth (edema), nodular (sarcoidosis, lymphangitic carcinomatosis), or calcified (healed infections).
- Position: Predominantly peripheral or basal zones in venous congestion; diffuse or upper lobe in sarcoidosis.
- Character: Smooth, nodular, or calcified.
- Time: Acute (pulmonary edema) to chronic (fibrosis or calcifications).
- Associated Findings: Crazy paving, ground-glass opacity, nodules.
- Other relevant Imaging Modalities:
- MRI: May identify fluid-related septal thickening.
- PET-CT: Highlights metabolic activity in malignancies.
- US: Detects associated pleural effusion.
- Angiography: Identifies vascular causes.
Labs:
- BNP for heart failure.
- Calcium and phosphate levels for calcifications.
- LDH for alveolar proteinosis.
- Biopsy or lavage for neoplastic or inflammatory causes.
Management:
- Treat underlying cause (e.g., diuretics for heart failure, chemotherapy for malignancies).
- Monitor for progression or complications.
Pulmonary function tests (PFTs):
Restrictive pattern in cases with widespread interstitial involvement.
Recommendations:
- Precise identification of the type and cause of thickening.
- Targeted management based on imaging and clinical findings.
Key Points and Pearls:
- Smooth thickening often indicates pulmonary venous congestion.
- Nodular thickening suggests neoplastic or granulomatous diseases.
- Calcified thickening points to chronic or healed conditions.
- Crazy paving is a pattern of interlobular thickening combined with ground-glass opacity, with a wide range of causes.
- Kerley B lines, though not commonly seen, are the most frequent manifestation of interstitial thickening in heart failure.
CHF

50 year old female with diabetes, chronic renal failure with congestive heart failure. CT in the coronal plane shows diffuse ground glass changes, Kerley B lines at the right base,
Ashley Davidoff MD TheCommonvein.net 50-003-CT

Ashley Davidoff MD TheCommonvein.net 50-004-CT

Ashley Davidoff MD TheCommonvein.net 50-010-CT

Ashley Davidoff MD TheCommonvein.net 50-007-CT
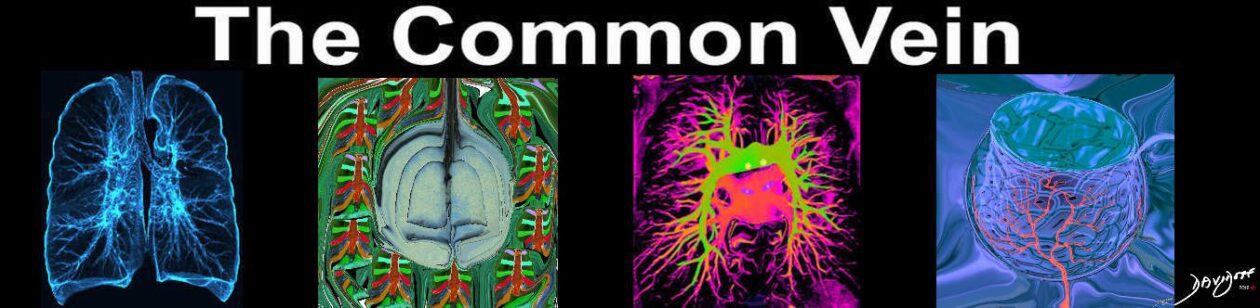
Amyloidosis
CT scan in the axial projection at the base of the lungs show many features of amyloidosis including lung nodules (white arrowheads) and infiltrates (b), and diffuse deposition within the alveolar septa (red arrowheads, c) and centrilobular nodules(yellow arrow c)
Ashley Davidoff MD Boston Medical Center
TheCommonVein.net septal-amyloidosis-001b
Nodular Thickening Along the Vein – Miliary TB

68 year old female presented with malaise night sweats weight loss Quantiferon gold positive, with a past history of treated TB in her native country as a child. Axial CT images through the upper lobe shows a miliary pattern of disease affecting interlobular septa, centrilobular and tree in bud nodular patterns. Bronchoscopy isolated Mycobacterium complex. She was treated with good result
Ashley Davidoff MD TheCommonVein.net mycobacterium-complex-TB-68-001

68 year old female presented with malaise night sweats weight loss QuantiFeron gold positive, with a past history of treated TB in her native country as a child. Axial CT images through the upper lobe shows a miliary pattern of disease affecting interlobular septa, centrilobular and tree in bud nodular patterns. Bronchoscopy isolated Mycobacterium complex. She was treated with good result
Ashley Davidoff MD TheCommonVein.net mycobacterium-complex-TB-68-008
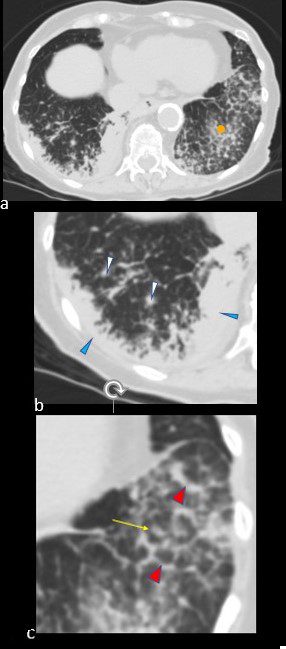
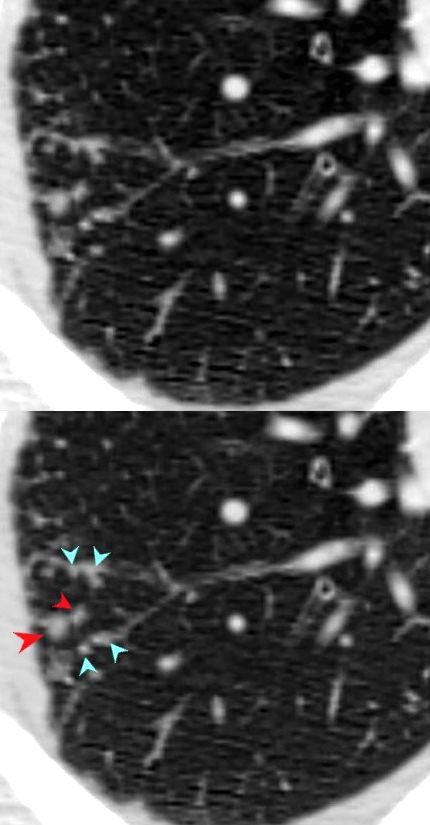
Sarcoidosis
SARCOIDOSIS, ACTIVE – ALVEOLAR FORM
SARCOIDOSIS, ACTIVE – ALVEOLAR FORM
48-year-old previously well presented with dyspnea and initial CXR showed an infiltrate at the right base, and clinically resolved. He presented a year later with right chest pain and low grade ever and the CXR showed patchy opacities in the LUL and in the RLL
A subsequent CT showed LUL nodular opacities and subpleural rim of consolidation in the LUL and more prominently at both lung bases, associated with significant mediastinal adenopathy. Lymphovascular nodularity was noted in the bronchovascular bundles as well as in the interlobular septa, consistent with sarcoidosis
CXR and CT 4 years later showed almost complete resolution of the parenchymal findings and the CT findings except for minimal reticulation and scarring in the subpleural regions
Ashley Davidoff MD
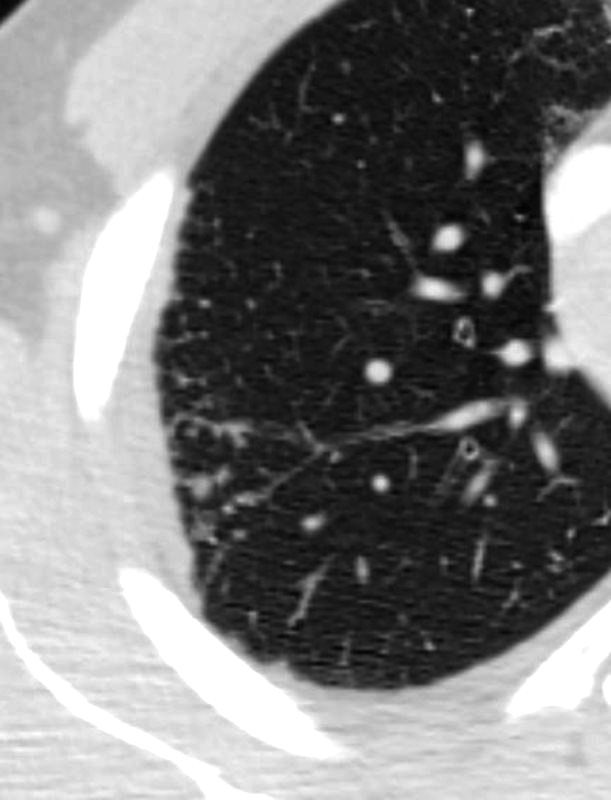
SARCOIDOSIS, ACTIVE – ALVEOLAR FORM
48-year-old previously well presented with dyspnea and initial CXR showed an infiltrate at the right base, and clinically resolved. He presented a year later with right chest pain and low grade ever and the CXR showed patchy opacities in the LUL and in the RLL
A subsequent CT showed LUL nodular opacities and subpleural rim of consolidation in the LUL and more prominently at both lung bases, associated with significant mediastinal adenopathy. Lymphovascular nodularity was noted in the bronchovascular bundles as well as in the interlobular septa, consistent with sarcoidosis
CXR and CT 4 years later showed almost complete resolution of the parenchymal findings and the CT findings except for minimal reticulation and scarring in the subpleural regions
Ashley Davidoff MD