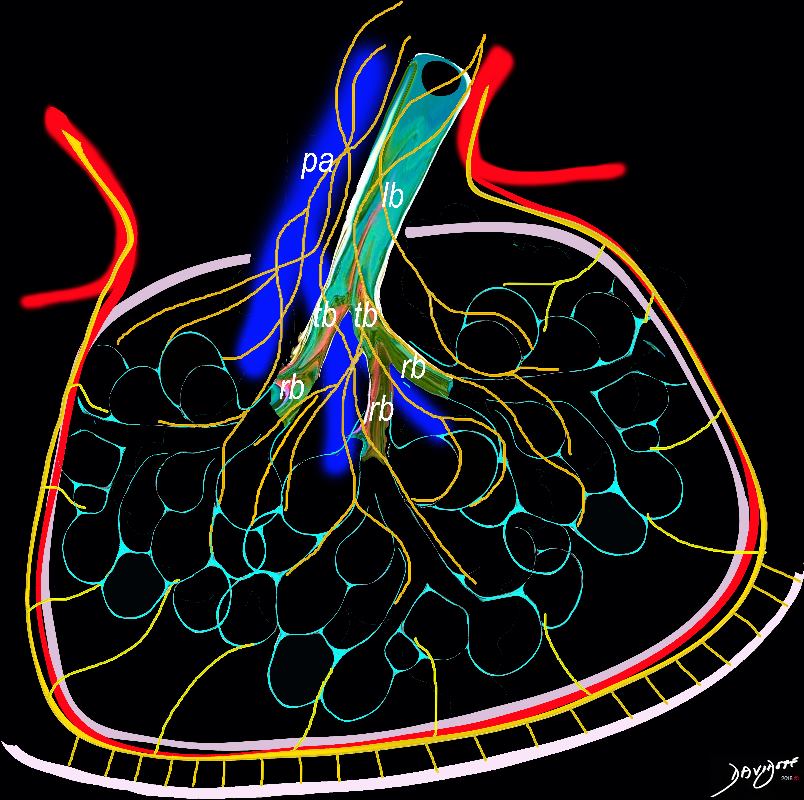
- Lymphatic system including ducts and nodes are distributed throughout the thoracic cavity, including around the lungs, bronchi, and along the major blood vessels.
- Pulmonary lymphatic vessels may thus be classified into
- Drains along the veins
- Superficial system
- pleural
- interlobular (in interlobular septa) and ,
- drains centrally along the veins to the hilum
- intralobular
- some of the drainage of the interstitium of the secondary lobule
- Deeper System
- intralobular.
- around
- bronchovascular bundle
- perivascular (associated with a blood vessel),
- peribronchiolar ( least prominent),
- interalveolar (in interalveolar septa)
- drains centrally along the bronchovascular bundle
- join the broncho-mediastinal trunks
- around
- intralobular.
- Lymph node enlargement can result from various diseases, categorized by their underlying etiology. The causes can be grouped into the following TCV disease categories:
- Infection:
- Tuberculosis (TB): A common cause of mediastinal lymphadenopathy, particularly in endemic areas. TB causes granulomatous inflammation of lymph nodes.
- Bacterial infections: Conditions like bacterial pneumonia, bronchitis, or infections like cat-scratch disease (Bartonella) and brucellosis can cause reactive lymphadenopathy.
- Viral infections:
- Epstein-Barr virus (EBV): Causes infectious mononucleosis, which can lead to generalized lymphadenopathy.
- HIV/AIDS: Can cause generalized lymphadenopathy, especially during acute infection or advanced stages of the disease.
- Cytomegalovirus (CMV): Another viral infection causing lymph node enlargement.
- Fungal infections: Histoplasmosis and coccidioidomycosis can cause enlarged lymph nodes, particularly in endemic areas.
- Inflammation:
- Sarcoidosis: A systemic granulomatous disease that often causes bilateral hilar lymphadenopathy. It involves non-caseating granulomas and affects multiple organs.
- Rheumatoid arthritis (RA): Can cause reactive lymphadenopathy as part of a systemic inflammatory response.
- Systemic lupus erythematosus (SLE): This autoimmune disease can cause generalized lymphadenopathy due to inflammation.
- Neoplasm:
- Lung cancer (primary and metastatic): Lymph node enlargement in the mediastinum or hilar regions is common, often as a result of metastatic disease. Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) can both spread to lymph nodes.
- Lymphoma: Includes Hodgkin’s lymphoma and non-Hodgkin lymphoma. These cancers often present with large, painless lymph nodes in the chest or supraclavicular regions.
- Metastatic cancer: Tumors from the breast, head and neck, esophagus, or other organs can spread to nearby lymph nodes, causing enlargement.
- Mechanical Causes:
- Obstruction: Compression or blockage of lymphatic drainage can result in localized lymph node enlargement. This can occur due to tumors or lymphatic vessel blockage.
- Chylothorax: Lymphatic fluid leakage into the pleural cavity can result in enlarged lymph nodes due to the accumulation of lymphatic fluid.
- Trauma:
- Post-surgical lymphadenopathy: After thoracic or chest surgery (e.g., lung biopsy, lymph node dissection), reactive enlargement of lymph nodes may occur as part of the healing process.
- Trauma: Injury to the chest or lymphatic vessels can result in localized swelling or inflammation of nearby lymph nodes.
- Metabolic:
- Gaucher’s disease: A lysosomal storage disorder that can cause enlargement of the spleen, liver, and lymph nodes due to accumulation of glucocerebrosides.
- Amyloidosis: The deposition of amyloid proteins in tissues, including lymph nodes, can lead to their enlargement.
- Circulatory:
- Congestive Heart Failure (CHF): Fluid congestion in the chest can cause reactive enlargement of lymph nodes due to lymphatic drainage impairment.
- Pulmonary edema: As a result of circulatory issues like left heart failure, edema may result in secondary lymph node enlargement due to lymphatic overload.
- Immune:
- HIV/AIDS: Can cause generalized lymphadenopathy, particularly in the early stages or advanced stages of the disease, due to immune system activation.
- Autoimmune diseases: Conditions like SLE, Sjogren’s syndrome, or vasculitis may lead to reactive lymph node enlargement due to immune system dysregulation.
- Inherited and Congenital:
- Primary immunodeficiencies: Such as Wiskott-Aldrich syndrome or hyper-IgM syndrome, may lead to persistent or recurrent infections, causing chronic lymph node enlargement.
- Lymphatic malformations: Congenital abnormalities in lymphatic development can result in abnormal lymph node enlargement and lymphatic drainage issues.
- Idiopathic:
- Idiopathic granulomatous disease: Some conditions, like Wegener’s granulomatosis or Churg-Strauss syndrome, may cause unexplained lymph node enlargement.
- Iatrogenic:
- Post-radiation lymphadenopathy: Lymph node enlargement may occur after radiation therapy for cancers such as lung cancer or lymphoma.
- Post-treatment lymphadenopathy: Medications, such as some antibiotics or antiepileptics, may cause lymphadenopathy as an adverse reaction.
- Functional:
- Reactive lymphadenopathy: A transient enlargement of lymph nodes due to functional responses from the immune system, often following mild infections or inflammatory processes.
- Psychological and Psychiatric:
- Psychological or psychiatric conditions do not typically directly cause lymph node enlargement. However, stress and chronic psychological conditions can affect immune function, possibly leading to secondary responses such as mild lymph node enlargement.
Next Step:
- Imaging evaluation:
- For suspected lymph node enlargement, CT or MRI of the chest is usually the next step to assess the size, location, and number of enlarged nodes, and to determine if there is associated disease (e.g., malignancy, infection).
- Biopsy: If the cause is unclear, a biopsy of the enlarged lymph node may be necessary for definitive diagnosis, especially in suspected malignancy or infections.
- Infection:
The final common pathway for all the lymphatic is via the thoracic duct which enters the left subclavian vein.
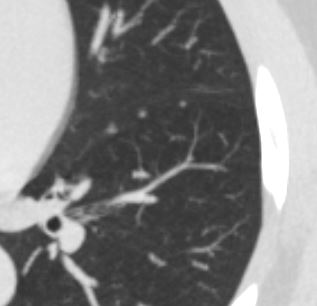
Cisterna Chyli
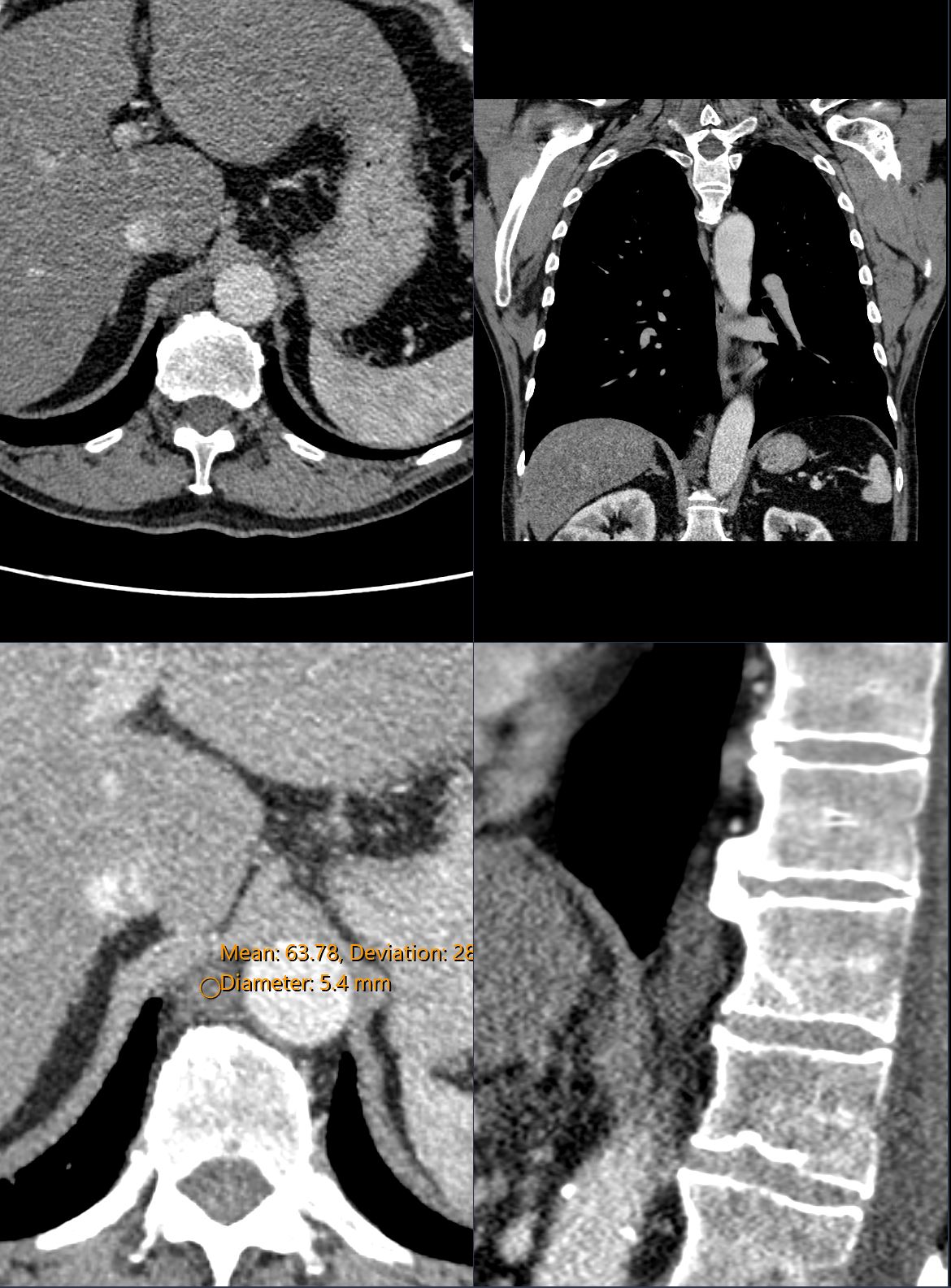
CT scan of the chest showing the cisterna chyli .
The cisterna chyli is the oval cystic structure located to the right of the aorta in the retrocrural space at the level of the L1-L2 vertebrae
It receives the lymphatic drainage from the abdomen, pelvis, and lower extremities and is considered the origin point of the thoracic duct, which travels upward to drain into the left subclavian vein at the base of the neck at the junction of the left subclavian vein and the left internal jugular vein. This is known as the left venous angle.
Ashley Davidoff TheCommonVein.net b11804
The Superficial and Deep Lymphatic Systems at the Secondary Lobular Level
The diagram shows the 2 systems of lymphatic drainage at the level of the secondary lobule. The superficial system drains some of the interstitium of the secondary lobule, runs in the interlobular septa and drains all the pleura. Thee pathway to the lymph nodes in the mediastinum is via the pulmonary veins. The deeper system drains the interstitium in the interalveolar septa, and then they travel along the bronchovascular bundle accompanying the bronchi and pulmonary artery and into the lymph nodes of the hila and mediastinum
Ashley Davidoff MD TheCommonVein.net lungs-0767
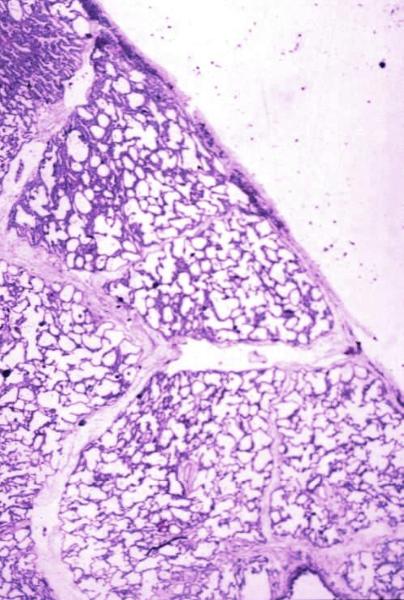
Normal lung histology
This image is a panoramic view of the lung showing secondary lobules and interlobular septa. Within the interalveolar septae, one sees small venules and lymphatics.Courtesy Armando Fraire MD. 32649b
code lung pulmonary alveoli alveolus secondary lobule interlobular septa vein lymphatic histology
interstitium interstitial
32649b
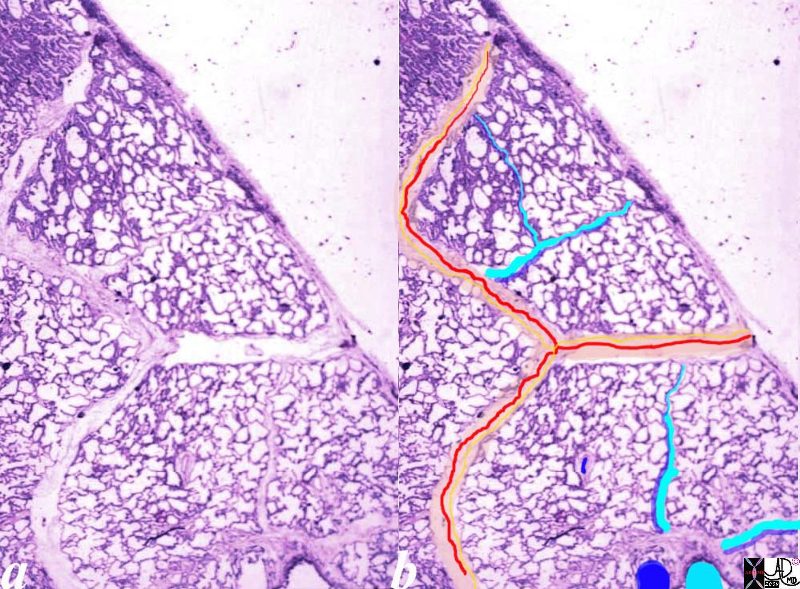
Normal lung histology
This image is a panoramic view of the lung showing secondary lobules and interlobular septa. Within the interalveolar septae, one sees small venules and lymphatics .
The side by side images show the interlobular septa within which reside the pulmonary venules (red) and lymphatics and within the center of the lobule run the respiratory bronchioles (teal) and pulmonary arterioles (blue)
Courtesy Armando Fraire MD. 32649b
key words
lung pulmonary alveoli alveolus secondary lobule interlobular septa vein lymphatic histology
interstitium interstitial
32649c06.8s
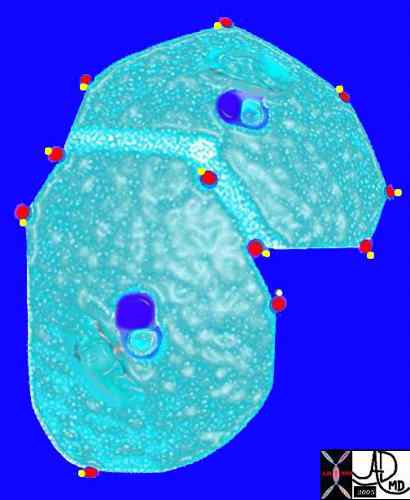
The arteries and airways pair up and travel together from the interlobular septa to the hilum. The pulmonary lobule, also called the secondary lobule is a structural unit surrounded by a membrane of connective tissue, and it is smaller than a subsegment of lung but larger than an acinus. This diagram shows two secondary lobules lying side by side. The pulmonary arteriole (royal blue) and bronchiole (pink) are shown together in the centre of the lobule (“centrilobular”), while the oxygenated pulmonary venules (red) and lymphatics (yellow) are peripheral and also form a formidable and almost inseparable pair.
42440b03
Davidoff Art Courtesy Ashley Davidoff MD
-
Categories
- Pulmonary Lymph Nodes: Found within the lung tissue and around the bronchi, they drain lymph from the lung parenchyma and airways.
- Hilar Lymph Nodes: Located at the hilum of each lung, these nodes receive lymph from the lung tissue and airways.
- Mediastinal Lymph Nodes: Located in the mediastinum (between the lungs), these nodes are involved in draining lymph from the lung hilum, the esophagus, the heart, and the trachea.
- Cervical Lymph Nodes: These are located in the neck and receive lymphatic drainage from the superior portion of the lungs, particularly from the upper lobe and trachea.
- Supraclavicular Lymph Nodes: Located just above the clavicle, these nodes can be involved in the spread of lung cancer or infections.
- Paratracheal Lymph Nodes: These nodes are located beside the trachea and drain lymph from the trachea, bronchi, and lungs.
Difference Between the Upper Lobes and Lower Lobes
- The lymphatic drainage of the upper lobes and lower lobes of the lungs differs
- In the upper lobes
- lymphatic drainage is more extensive and interconnected
- because the
- upper lobes contain a
- larger number of lymph nodes
- lymphatic vessels in the upper lobes also tend to be
- larger and
- more numerous and therefore
- more efficient circulation of lymphatic fluid.
- the lower lobes of the lungs
- limited lymphatic drainage system,
- fewer lymph nodes and
- smaller lymphatic vessels.
- upper lobes contain a
- In the upper lobes
Lymph Nodes on the Fissures
Ashley Davidoff MD TheCommonVein.net

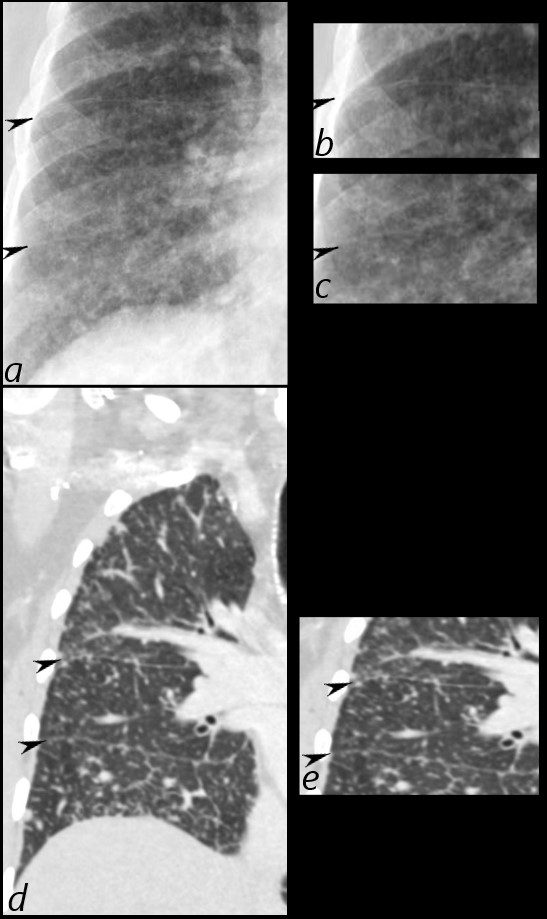
In these images. and c are normal and b and d represent thickened interlobular septa in a patient with congestive heart failure. These are the well known Kerley lines, often spoken about but rarely seen. They are identified as thin horizontal lines usually seen in the costophrenic angles, not being longer than 2 cms in length and touching the pleural surface.
Ashley Davidoff MD TheCommonVein.net
42545c01

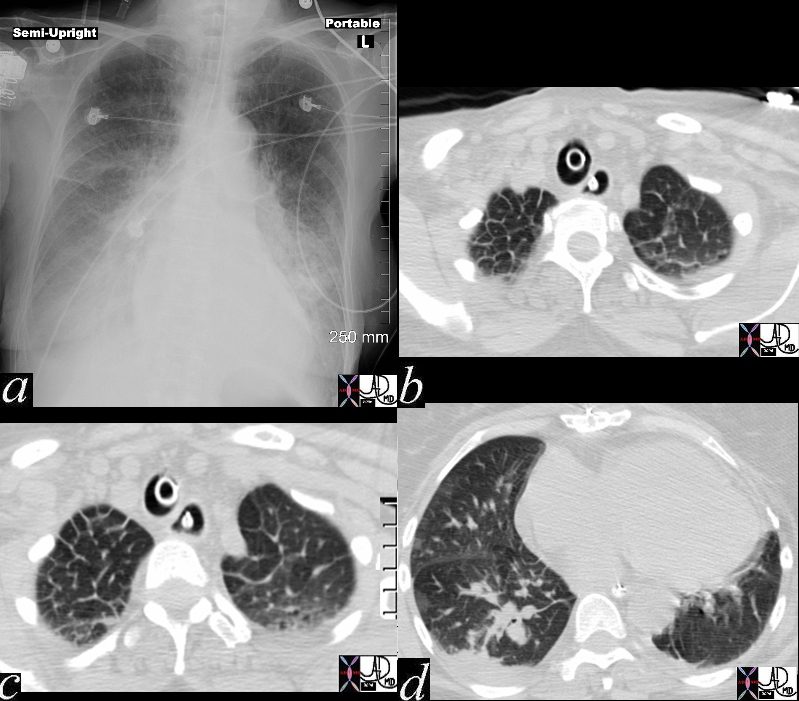
There is severe congestive cardiac failure in this CXR with evidence of Kerley B lines seen as horizontal thin lines touching the pleura in the right costophrenic angle in a, and the presence of 3 thin lines coursing obliquely toward the hilum in image b, representing distended lymphatics running with the bronchovascular bundles. These lines are called Kerley A lines and they are quite rare. Kerley C lines represent the reticular pattern of intraparenchymal lymphatics which in this case are quite vague.
46424c01
Ashley Davidoff MD TheCommonVein.net
Keywords
heart cardiac chest fx pulmonary congestion interstitial edema enlarged pulmonary arteries pulmonary arteriole to bronchiole ratio increased cardiomegaly fx enlarged Kerley B line thickened interlobular septa dx congestive heart failure CHF cardiac failure CTscan CXR plain film Davidoff MD 46425 46427 46428 46432c01 46431 46432 46432c0146424c01
Ashley Davidoff MD TheCommonVein.net
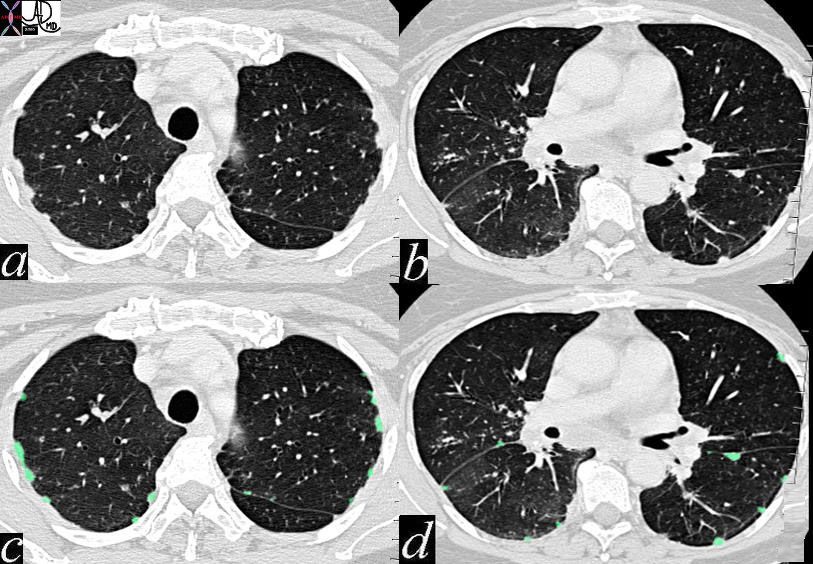
keywords lung pleura fissures lymphatics interstitium interstitial disease fx nodules dx sarcoidosis CTscan 446843c01 6842 46843 46843c01 46846 46847 46848 46849 46851
Ashley Davidoff MD TheCommonVein.net
Halo Sign Around a Malignant Mass and
Lymphangitis Carcinomatosis
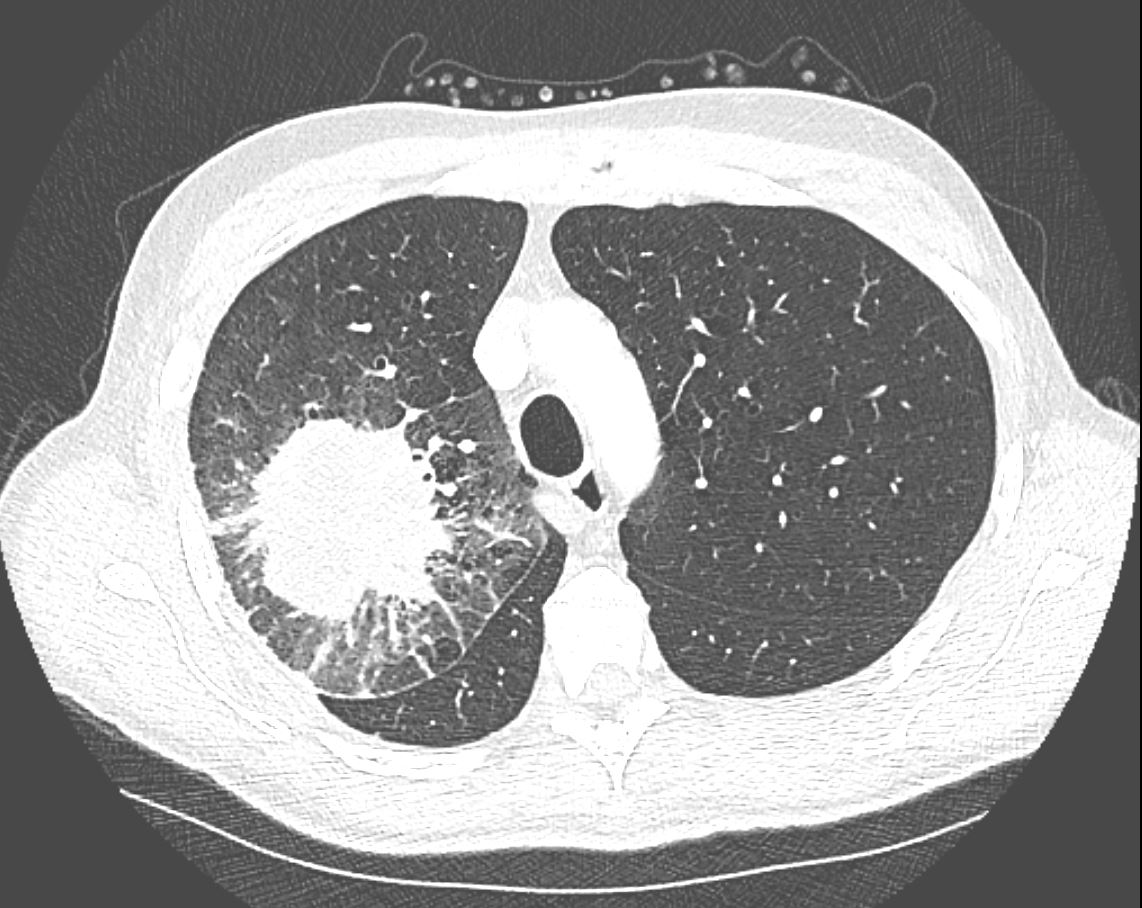

CT in the axial plane demonstrates a large, spiculated mass in the right upper lobe with surrounding halo likely reflecting hemorrhage or lymphatic edema around the mass. In addition, there is evidence of irregular interlobular septal thickening likely reflecting lymphatic invasion and indicating lymphangitis carcinomatosa. There is irregular thickening of the major fissure suggesting involvement.
Ashley Davidoff MD TheCommonVein.net 135865
Lymph Nodes
Most of the visible lymph nodes are within the hila and mediastinum. However there are lymph nodes that lie close to the periphery of the lung. These are relatively small measuring approximately 2mm. in diameter. They become larger towards the hila, reaching diameters of between 5 to 10mm.
The mediastinal nodes have been divided into 4 main groups; the superior mediastinal, aortic, inferior mediastinal, and N node are the designated groups. Within these groups there are 14 nodal stations. These 14 stations have been given both names and numbers to aid in the classification and staging of disease.
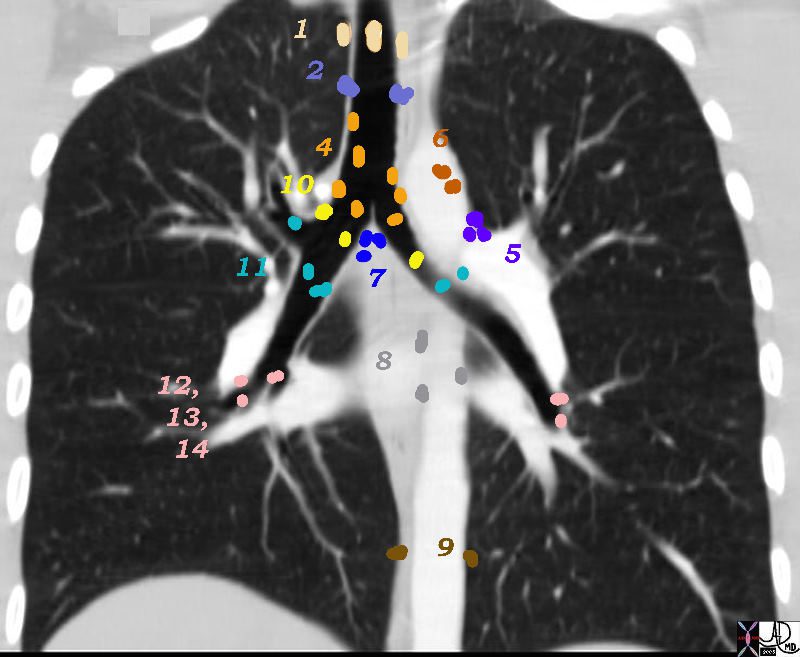

There are Aortic Nodes 5 Subaortic Nodes (A-P window) 6 Paraaortic Nodes (Ascending Aorta or Phrenic) Inferior Mediastinal Nodes 7= subcarinal nodes 8=Paraesophageal Nodes 9 = Pulmonary Ligament Nodes 10 Hilar Nodes 11 Interlobar Nodes 12 Lobar Nodes
Ashley Davidoff
TheCommonVein.net
32682n04.801
Group 3 called “prevascular and retrotracheal nodes” can only be appreciated on the axial images since they are found relatively anterior and posteriorly in the chest.
Superior Mediastinal Nodes
1 Highest Mediastinal Nodes
2 Upper Tracheal Nodes
3 Prevascular and Retrotracheal Nodes
4 Lower Paratracheal Nodes
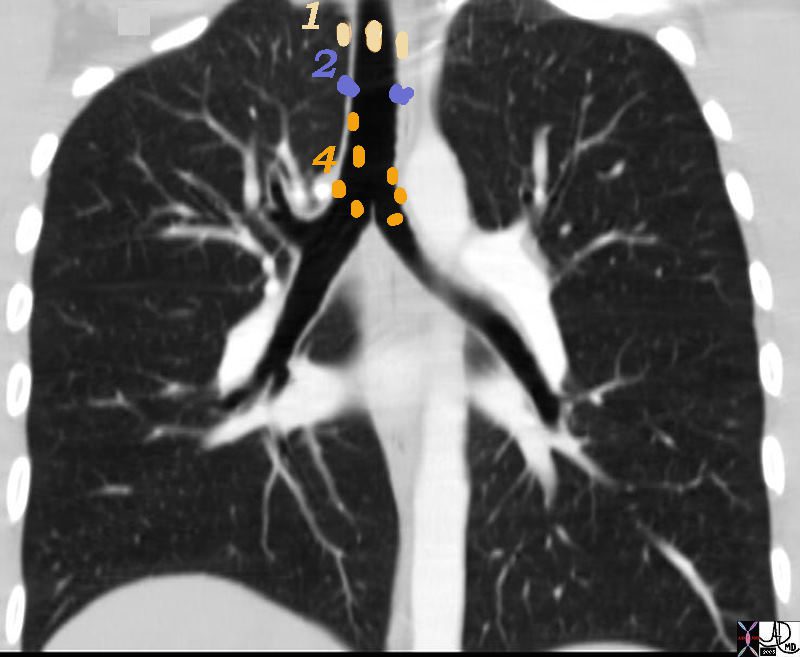

1= Highest, 2= Upper Tracheal and 4 = Lower Paratracheal
32682n01n.800 nodes 1,2,4 Superior mediastinal Nodes 1 Highest Mediastinal Nodes 2 Upper Tracheal Nodes 3 Prevascular and Retrotracheal Nodes 4 Lower Paratracheal Nodes chest mediastinum lymph nodes normal anatomy CTscan Ashley Davidoff MD TheCommonVein.net
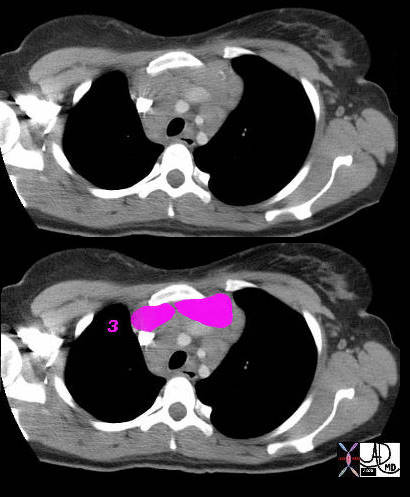

3 = Prevascular Nodes
This patient has lymphoma and the nodal groups of the mediastinum are all enlarged.
42058c01b
Ashley Davidoff MD TheCommonVein.net
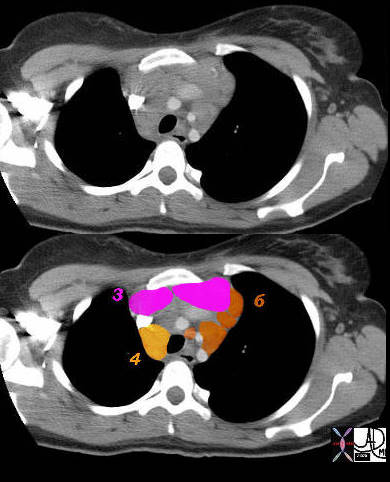

3 = Prevascular 4 = Lower Paratracheal
Aortic Nodes
6= Paraaortic Nodes
42058c02 Ashley Davidoff MD TheCommonVein.net
Aortic Nodes
5 Subaortic Nodes (A-P window)
6 Paraaortic Nodes (Ascending Aorta or Phrenic)
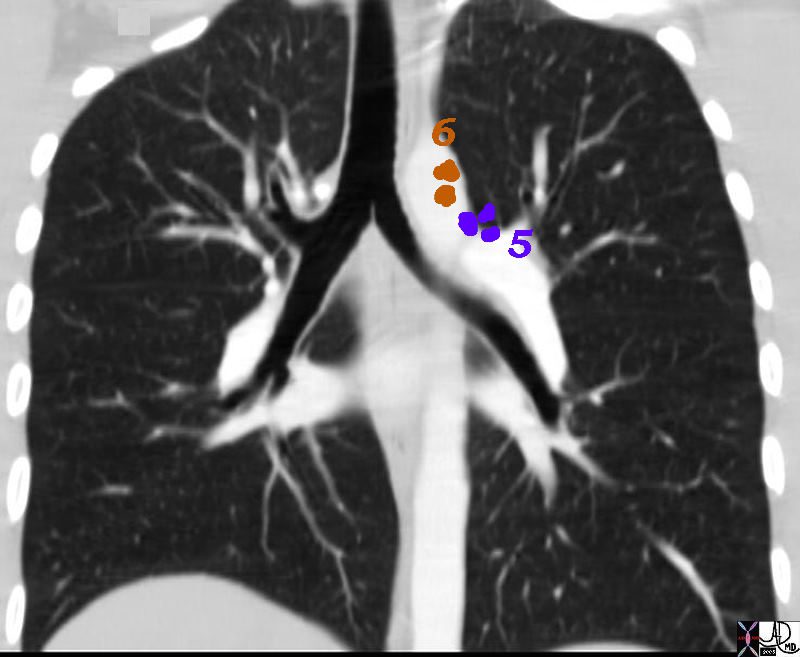

5= A-P window and 6= Paraaortic
32682n07n.800 Ashley Davidoff MD TheCommonVein.net
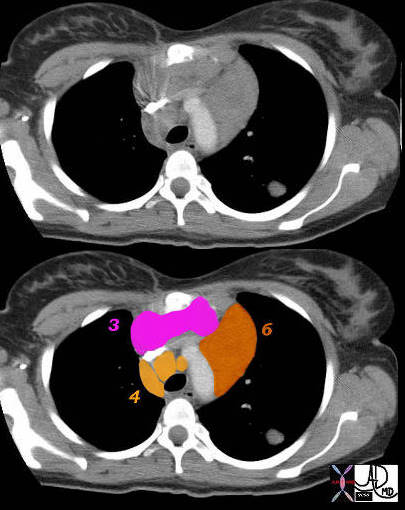

6= paraaortic
Superior Mediastinal
3 – Prevascular Nodes 4 = Lower Paratracheal
42059c01 Ashley Davidoff MD TheCommonVein.net
Inferior Mediastinal Nodes
7 Subcarinal Nodes
8 Paraesophageal Nodes
9 Pulmonary Ligament Nodes
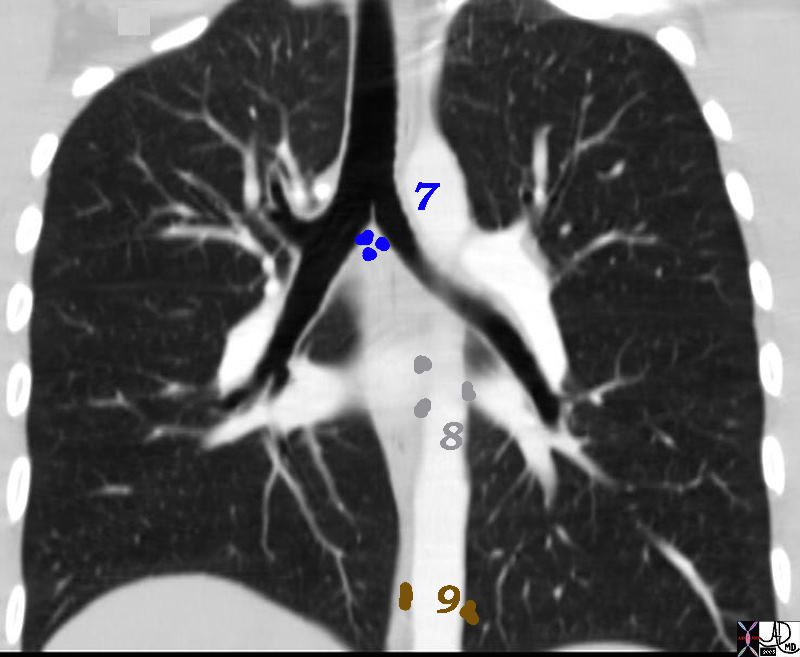

7= Subcarinal, 8= Paraesophageal and 9= Pulmonary Ligament
32682n06n.800 Ashley Davidoff MD TheCommonVein.net
10 Hilar Nodes
11 Interlobar Nodes
12 Lobar Nodes
13 Segmental Nodes
14 Subsegmental Nodes
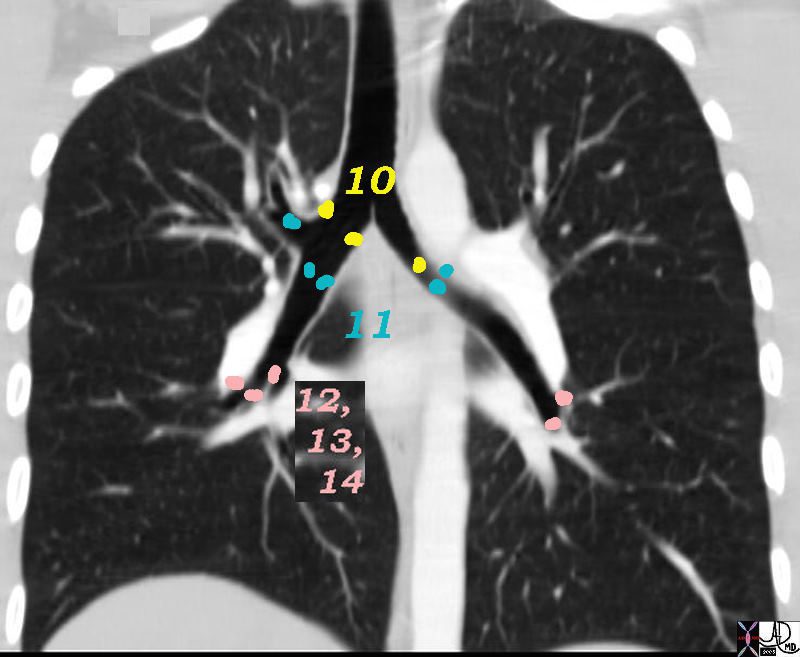

10=Hilar, 11=”Interlobar,” 12=”Lobar,” 13=”Segmental,” 14 = Subsegmental
32682n05ns.800 Ashley Davidoff MD TheCommonVein.net



keywords lung pleura fissures lymphatics interstitium interstitial disease fx nodules dx sarcoidosis CTscan 446843c01 6842 46843 46843c01 46846 46847 46848 46849 46851
Ashley Davidoff MD TheCommonVein.net

keywords lung pleura fissures and around the bronchi
key words lymphatics interstitium interstitial disease fx nodules dx sarcoidosis CTscan 446843
Ashley Davidoff MD TheCommonVein.net

Patient with sarcoidosis showing mediastinal lymphadenopathy in the aortic nodes paraaortic 6, A-P window 5, Inferior mediastinal subcarinal 7, N Nodes hilum 10, interlobar 11, segmental 13 n Ashley Davidoff MD TheCommonVein.net 46851c01


A Patient with Sarcoidosis with Calcified Nodes at Many Stations
50-year-old male presents with history of Stage 4 sarcoidosis acute chest pain and dyspnea
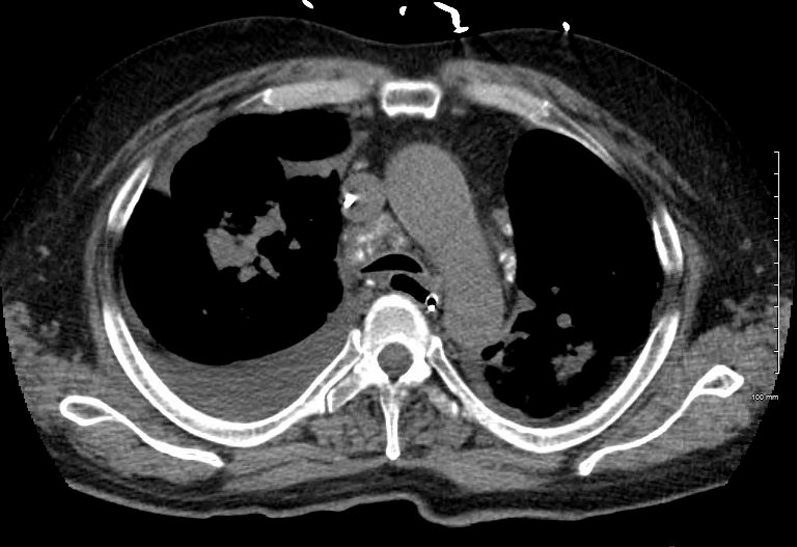
The initial CXR shows a left sided pneumothorax, diffuse nodular pattern with confluent perihilar infiltrates and a left pleural effusion
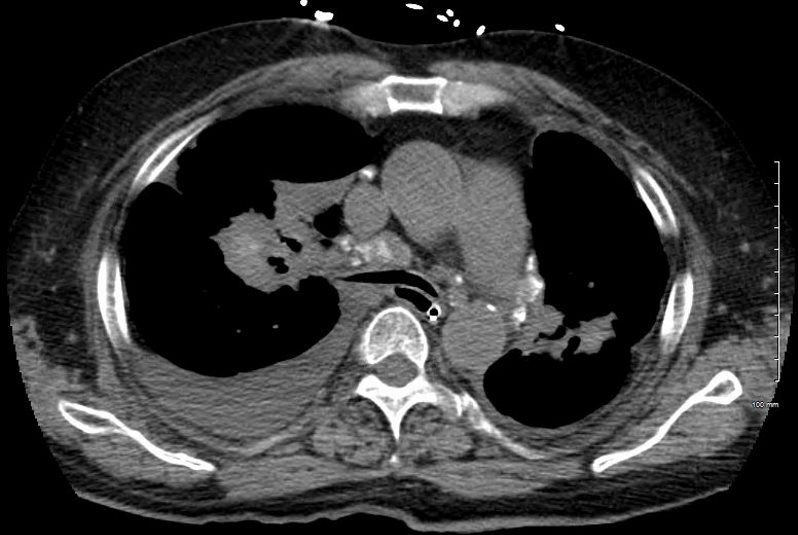
A chest tube was placed and a chest CT showed confluent fibrotic masses in the hilar regions totally surrounding the bronchovascular bundles with encasement of the middle lobe artery. In addition, multiple lympho-vascular micronodules are demonstrated. The pulmonary artery measures 32.7mm indicating pulmonary hypertension.
A CXR during this admission shows re-expansion of the pneumothorax. Left lung volume is reduced.
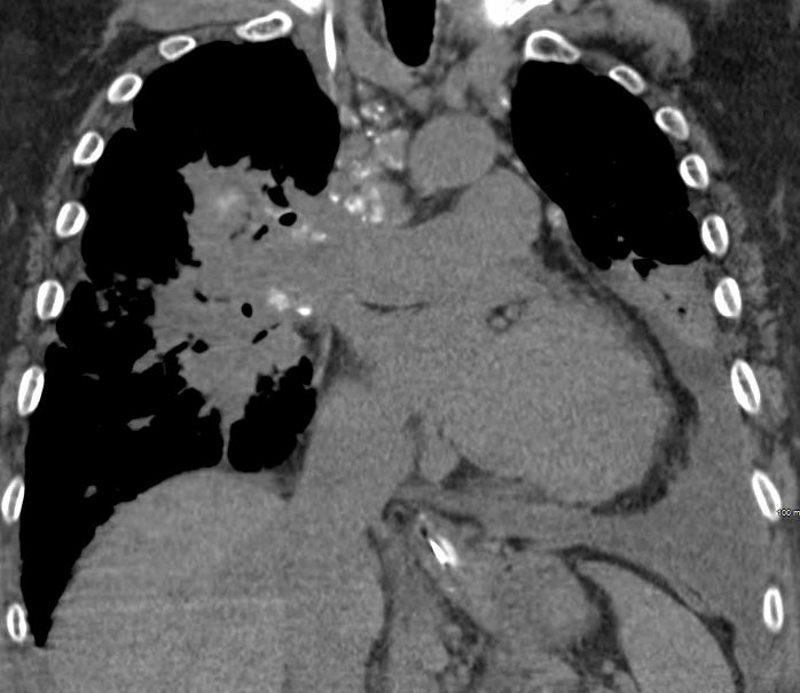
The patient presents 2 years later, again with progressive dyspnea and chest pain and CT PA shows encasement of the airways, right middle lobe pulmonary artery and left lower pulmonary vein by the fibrotic broncho vascular masses, and non-occlusive, subacute pulmonary embolus of the LPA. There are moderate bilateral pleural effusions, calcified lymph nodes, with ongoing pulmonary hypertension with right ventricular enlargement, right atrial enlargement, tricuspid regurgitation and pulmonary hypertension. At this time the patient is intubated.
He again presents 1 month after with chest pain and dyspnea. At this time, he has a tracheostomy. The scout frontal view shows persistent encasement of the left upper lobe bronchus and significant reduction on the volume of the left lung with elevated left hemidiaphragm.
CT PA has similar findings with a large right pleural effusion and unresolved large non occlusive thrombus in the left pulmonary artery.


SARCOIDOSIS, STAGE IV, PTX, ENCASEMENT
Ashley Davidoff MD

Ashley Davidoff MD



SARCOIDOSIS, STAGE IV, PTX, ENCASEMENT
Ashley Davidoff MD




Ashley Davidoff MD

SARCOIDOSIS, STAGE IV, PTX, ENCASEMENT
Ashley Davidoff MD

Ashley Davidoff MD




Ashley Davidoff MD


Ashley Davidoff MD

Ashley Davidoff MD


Ashley Davidoff MD

Ashley Davidoff MD

Ashley Davidoff MD

Ashley Davidoff MD
See Radiographics
Applied Anatomy
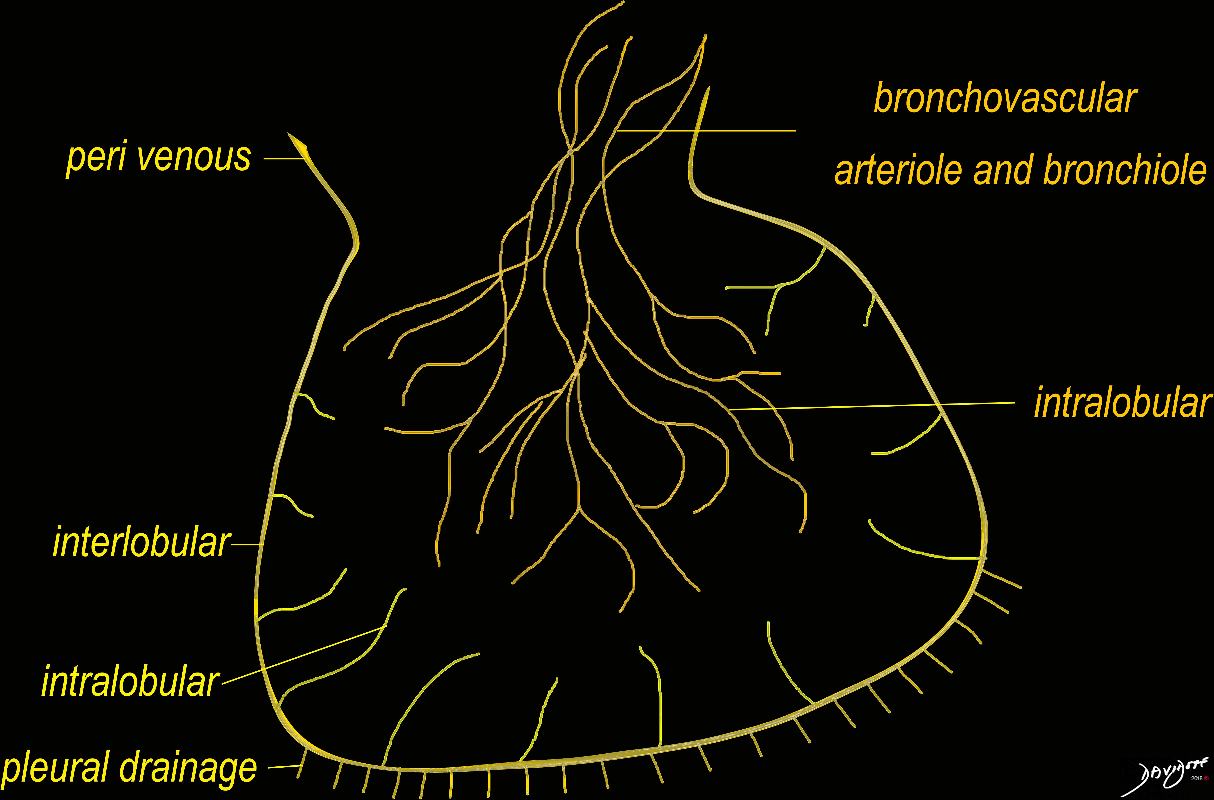
Knowledge of the anatomy of the secondary lobule is key to the understanding of the subpleural lymphatic system since the lymphatic of this region run in the interlobular septa. The anatomy of the secondary lobule was extensively discussed in part 1 of the lung module. The ability to image the secondary lobule is key to the diagnosis of many of the interstitial lung diseases and it requires high resolution imaging to enable distinction between changes in the interlobular septa changes within the central bronchovascular bundle and changes within the lobule itself. Since the subpleural lymphatics run in the interlobular septa, it becomes our focus when lymphatic disease is evaluated.
Certain diseases have a predilection for the lymphatic system at the subpleural level including sarcoidosis. On the other hand diseases such as lung carcinoma have a predilection for the deep and central nodal system. Since the systems do connect and are both usually involved i is imperative to evaluate both systems.
Subpleural System
The polygonal shape of the secondary lobule is a key shape to recognise. These lobules are well formed at the lung apices, lung bases and particularly at the periphery of the lung. The septa are not usually appreciated in healthy lungs, but may be seen in only mildly diseased lungs as well as advanced disease in the lung. Their presence does not necessarily indicate lymphatic disease since the connective tissue of the septa, and the venules are also located within them.
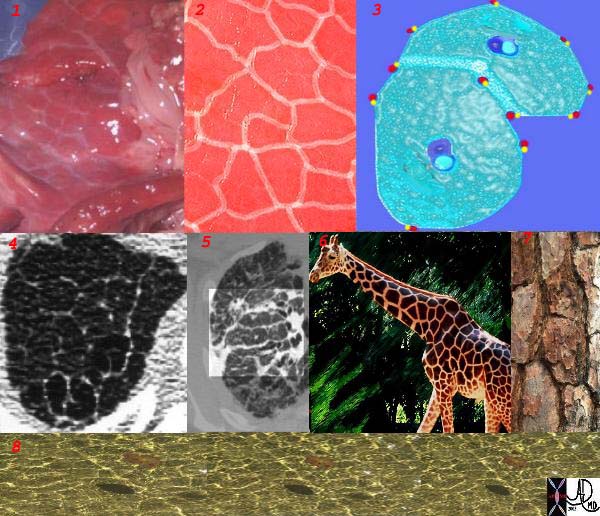


This is a series of images demonstrating the shape of the secondary lobule. The first image (1) is a post mortem specimen with congested lungs showing the interlobular septa, while the next (2), is an overlay of the septa in white showing their polygonal shape. The next drawing reveals side-by-side secondary lobules with central bronchovascular bundles and peripheral lympho-vascular bundles. Image 4 is a CT image through the apex of the lung showing thickened secondary lobules in a patient with mild emphysema, and 5 shows marked thickening of the interlobular septa in a patient with end stage sarcoidosis. 6,7,8 show the shape of the secondary lobules in the skin of a giraffe, the bark of a pine, and the ripples of the water respectively.
Ashley Davidoff MD TheCommonVein.net 31866collageSarcoid disease and sarcoid nodules specifically, seek out the lymphatics of both the subleral and the deep systems. Thus when nodules or focal changes are identified on the pleural surfaces, including the fissures, interlobular septa, and bronchovascular bundles then sarcoidosis is a prime suspect. Pleural effusions on the other hand are distinctly uncommon in sarcoidosis.(1-4%). In lymphangitic spread of disease, malignant cells get bundled in to the lymphatics causing obstruction and reducing pulmonary capacity. Thickening of the interlobular septa is a characteristic finding in these cases. In anthracosis, lymph nodes and lymphatics get filled with carbon colored soot.
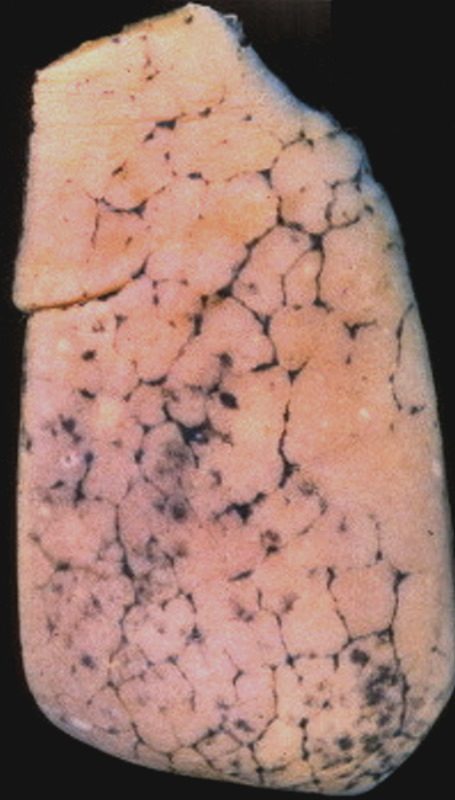

Anthracosis – Note the accumulation of carbon particles within the lymphatics along the interlobular septa, outlining the secondary lobules. The carbon particles are inhaled from an anthracotic urban environment. Courtesy Ashley Davidoff MD. 32291 code lung interlobular septum septa secondary lobule pulmonary lobule interstitium interstitial gross pathology carbon



This is a series of images demonstrating the shape of the secondary lobule. The first image (1) is a post mortem specimen with congested lungs showing the interlobular septa, while the next (2), is an overlay of the septa in white showing their polygonal shape. The next drawing reveals side-by-side secondary lobules with central bronchovascular bundles and peripheral lympho-vascular bundles. Image 4 is a CT image through the apex of the lung showing thickened secondary lobules in a patient with mild emphysema, and 5 shows marked thickening of the interlobular septa in a patient with end stage sarcoidosis. 6,7,8 show the shape of the secondary lobules in the skin of a giraffe, the bark of a pine, and the ripples of the water respectively.
Ashley Davidoff MD TheCommonVein.net 31866collage

Ashley Davidoff MD
TheCommonVein.net
32226c1
keywords
lungs pulmonary neoplasm primary prostate lymphatics distended metastases lymphangitis histopathology
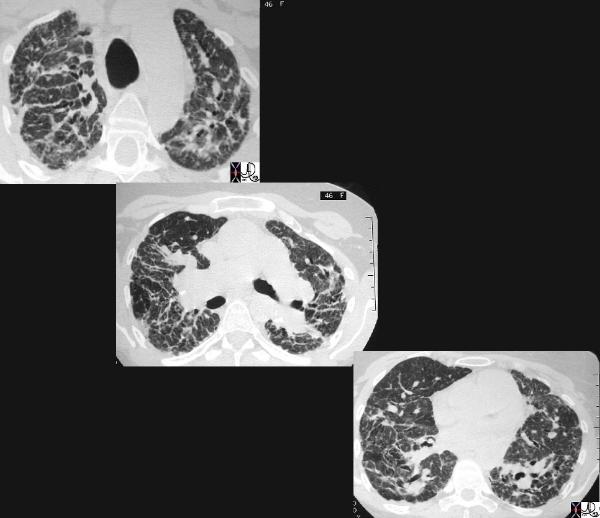

This cross sectional series of 3 CT images shows end stage sarcoidosis characterised by marked thickening along the lymphovascular bundles. The first image in the upper left shows marked thickening of the interlobular septa caused by granulomatous changes along the lymphatics. Courtesy Priscilla Slanetz MD. 31866c
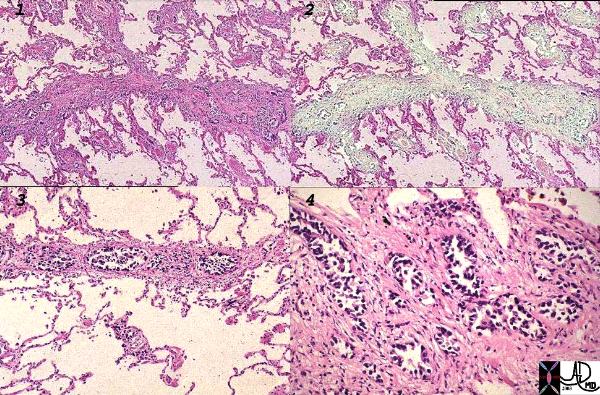
The Deep System
The subpleural and deep system have a final common pathway. The image below shows this connection by demonstrating spread of malignant disease in the interlobular septa, around the bronchi and in an intrapulmonary lymph node.
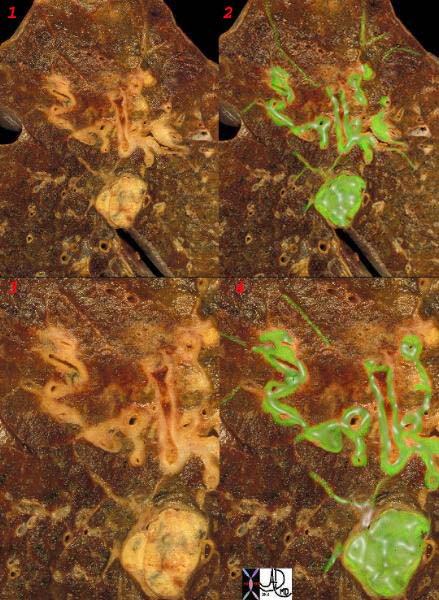

Ashley Davidoff MD
TheCommonVein.net
32199cw
keywords
lungs pulmonary parenchymal mass neoplasm primary lymphatics distended metastases lymphangitis gross pathology
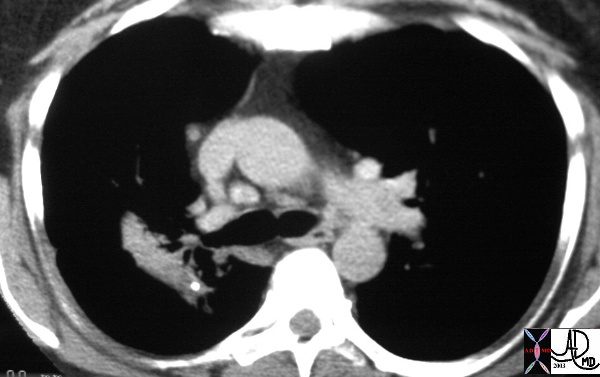

This patient with Stage III sarcoidosis has a calcified intraparenchymal node in the confluent fibrosis on the right.
Ashley Davidoff MD TheCommonVein.net 32001
Mediastinal Nodes
Size of Nodes
In general , although we often measure the size, and specifically the short axis of the nodes to determine the presence of disease, we understand that this is a fairly inaccurate method with low specificity . A short axis of more than 10mm implies a pathologically involved node. Often the large node may be reactive and may not contain malignant disease. PET scanning has been an important advance to aid in the distinction between reactive and malignant lymphadenopathy.
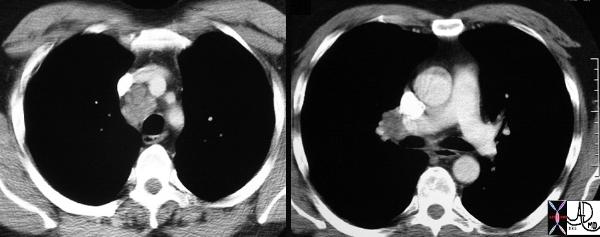

Courtesy Ashley Davidoff MD TheCommonVein.net 31646c
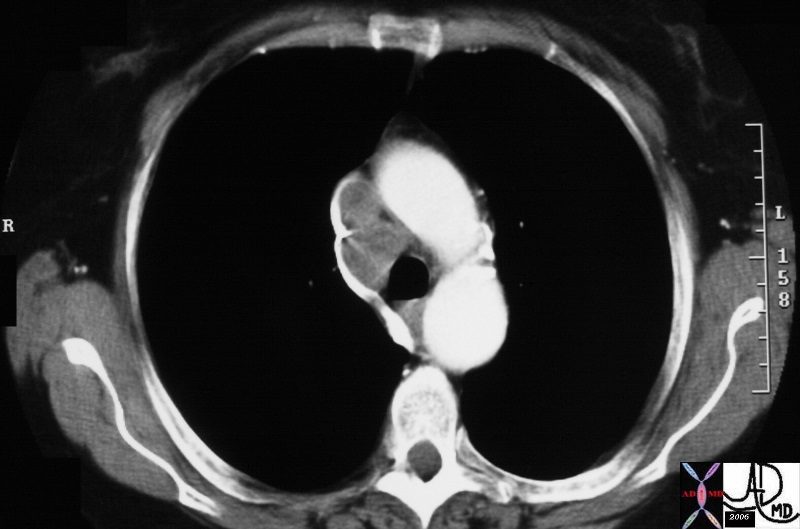

The node in the azygos region is pathologically enlarged but at pathology was shown to be reactive. Note the subtle deformity of the azygos region on the CXR below
33080.800 Courtesy Ashley Davidoff MD TheCommonVein.net
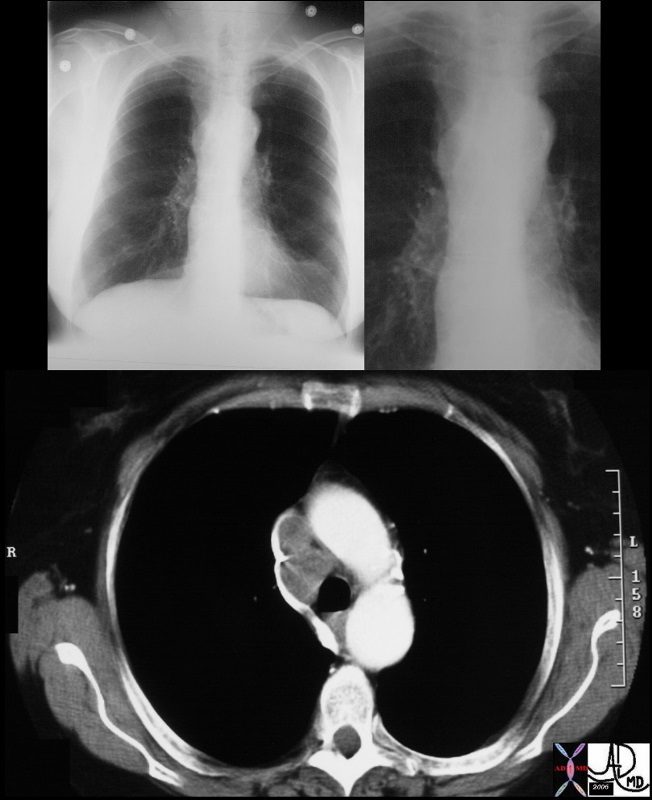

The node in the azygos region is pathologically enlarged but at pathology was shown to be reactive. note the subtle deformity of the azygos region on the CXR
33082c01 Courtesy Ashley Davidoff MD TheCommonVein.net
Large Reactive Node – Pathology negative
The node in the azygos region is pathologically enlarged but at pathology was shown to be reactive. note the subtle deformity of the azygos region on the CXR
33082c02 Courtesy Ashley Davidoff MD TheCommonVein.net
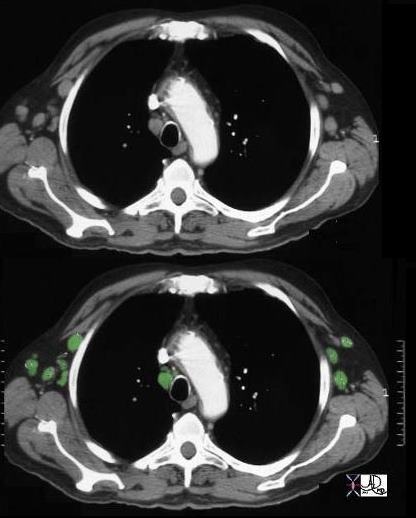

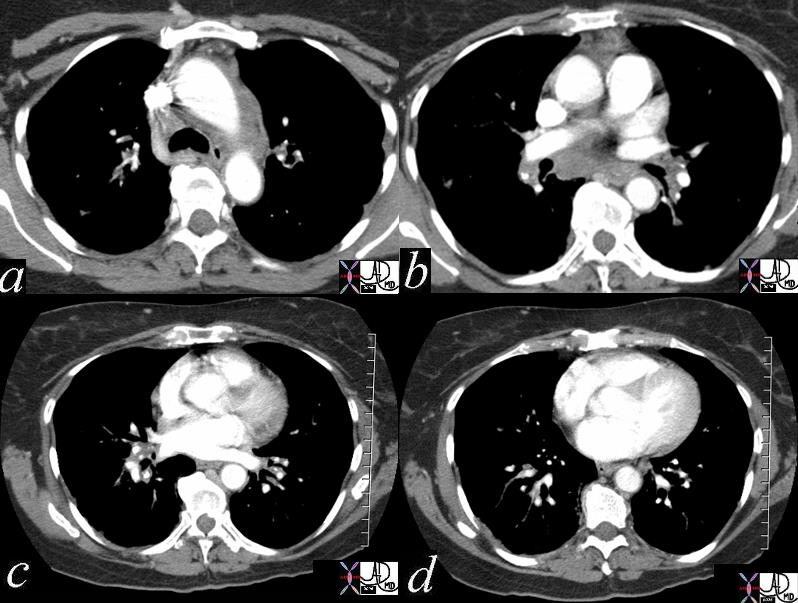
Patient with a history of lymphoma showing numerous enlarged lymph nodes in the mediastinum and axillae
32991b.800 Ashley Davidoff MD TheCommonVein.net
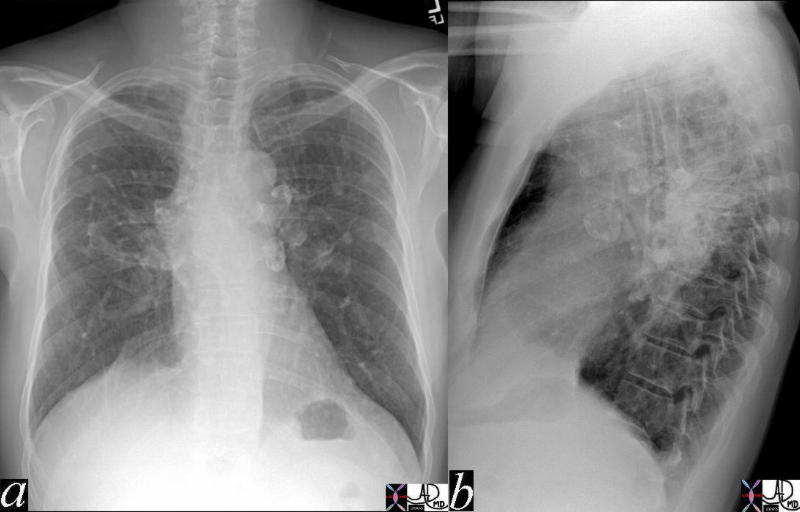

The A-P and lateral view of the chest is from a patient with sarcoidosis showing classical egg shell calcification of the mediastinal nodes and hilar nodes.
Ashley Davidoff MD TheCommonVein.net 42195c01
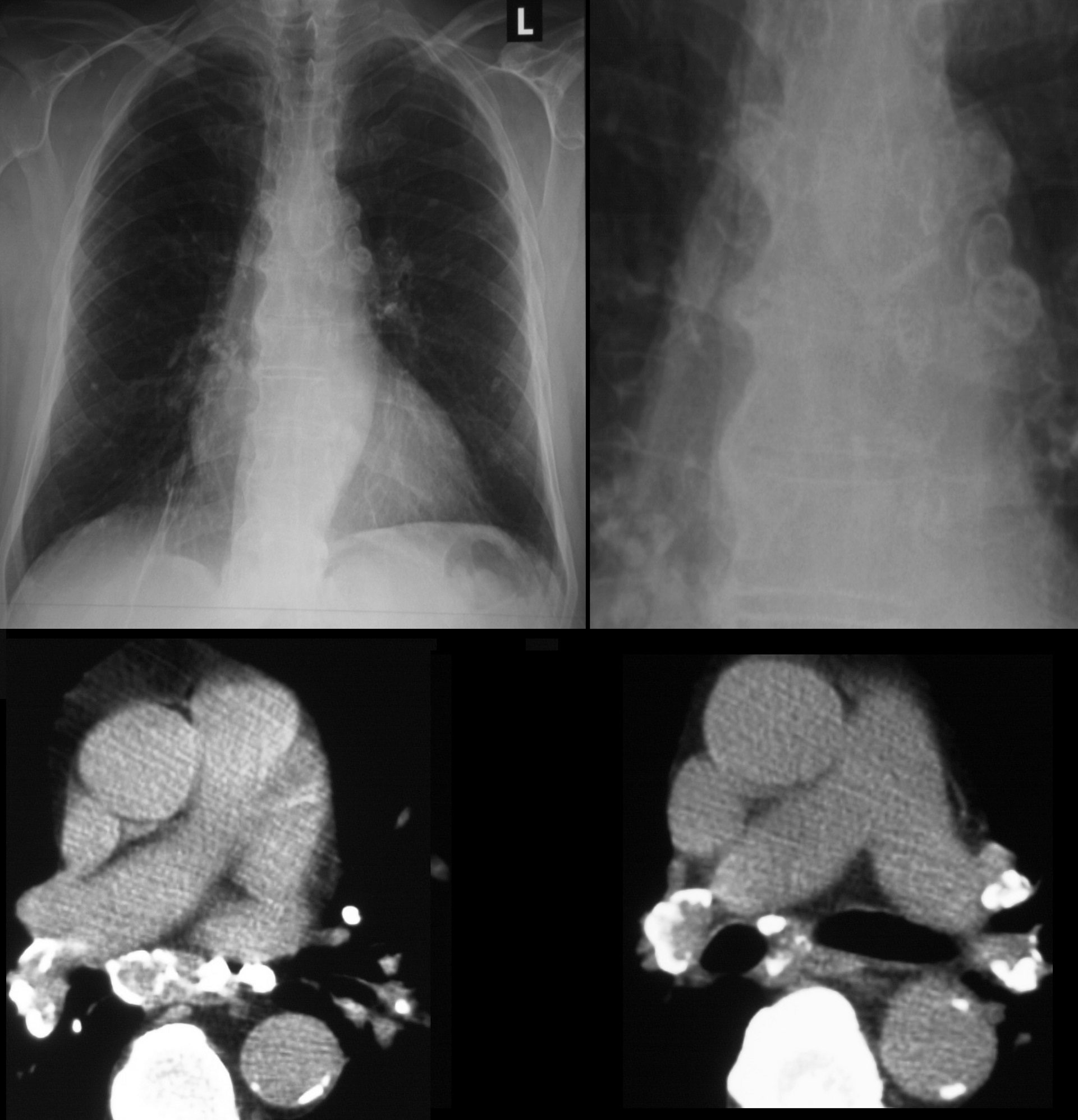

79 year old male with sarcoidosis and egg shell calcification of the hilar and mediastinal nodes
Ashley Davidoff MD TheCommonVein.net
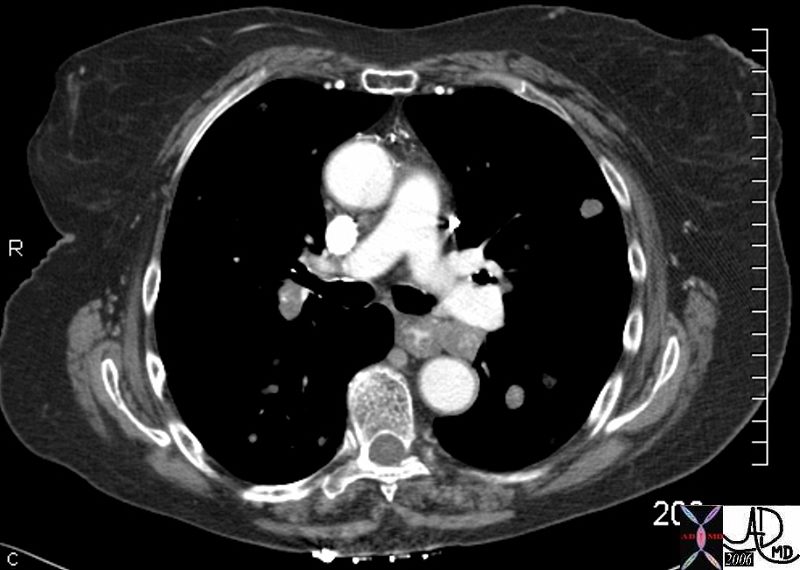

45137 elderly female with known sigmoid colon carcinoma liver lungs mediastinum hilar lymph nodes in right hilum fx calcified metastases fx calcifications A-P window node fx mediastinal lymphadenopathy Note that the CTscan was performed 2 years after the PET dx colonic mucinous adenocarcinoma with metastatic disease to the liver, mediastinal lymph nodes and lungs CTscan Ashley Davidoff MD TheCommonVein.net
45132 45133 45134 45135 45136 45139 45141
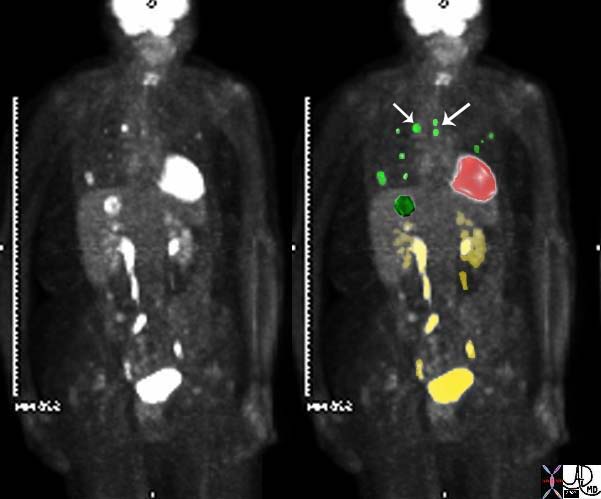

45137 elderly female with known sigmoid colon carcinoma liver lungs mediastinum hilar lymph nodes in right hilum fx calcified metastases fx calcifications A-P window node fx mediastinal lymphadenopathy Note that the CTscan was performed 2 years after the PET dx colonic mucinous adenocarcinoma with metastatic disease to the liver, mediastinal lymph nodes and lungs CTscan Ashley Davidoff MD TheCommonVein.net
45132 45133 45134 45135 45136 45139 45141
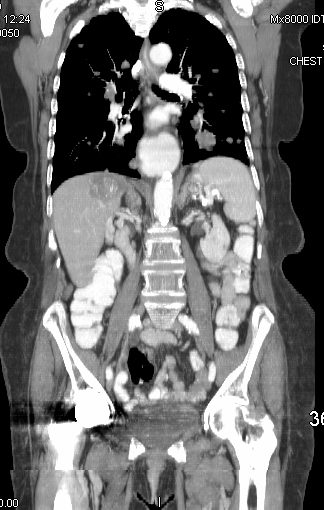

45137 elderly female with known sigmoid colon carcinoma liver lungs mediastinum hilar lymph nodes in right hilum fx calcified metastases fx calcifications A-P window node fx mediastinal lymphadenopathy Note that the CTscan was performed 2 years after the PET dx colonic mucinous adenocarcinoma with metastatic disease to the liver, mediastinal lymph nodes and lungs CTscan Ashley Davidoff MD TheCommonVein.net
45132 45133 45134 45135 45136 45139 45141

45137 elderly female with known sigmoid colon carcinoma liver lungs mediastinum hilar lymph nodes in right hilum fx calcified metastases fx calcifications A-P window node fx mediastinal lymphadenopathy Note that the CTscan was performed 2 years after the PET dx colonic mucinous adenocarcinoma with metastatic disease to the liver, mediastinal lymph nodes and lungs CTscan Ashley Davidoff MD TheCommonVein.net
45132 45133 45134 45135 45136 45139 45141

45137 elderly female with known sigmoid colon carcinoma liver lungs mediastinum hilar lymph nodes in right hilum fx calcified metastases fx calcifications A-P window node fx mediastinal lymphadenopathy Note that the CTscan was performed 2 years after the PET dx colonic mucinous adenocarcinoma with metastatic disease to the liver, mediastinal lymph nodes and lungs CTscan Ashley Davidoff MD TheCommonVein.net
45132 45133 45134 45135 45136 45139 45141

45137 elderly female with known sigmoid colon carcinoma liver lungs mediastinum hilar lymph nodes in right hilum fx calcified metastases fx calcifications A-P window node fx mediastinal lymphadenopathy Note that the CTscan was performed 2 years after the PET dx colonic mucinous adenocarcinoma with metastatic disease to the liver, mediastinal lymph nodes and lungs CTscan Ashley Davidoff MD TheCommonVein.net
45132 45133 45134 45135 45136 45139 45141

45137 elderly female with known sigmoid colon carcinoma liver lungs mediastinum hilar lymph nodes in right hilum fx calcified metastases fx calcifications A-P window node fx mediastinal lymphadenopathy Note that the CTscan was performed 2 years after the PET dx colonic mucinous adenocarcinoma with metastatic disease to the liver, mediastinal lymph nodes and lungs CTscan Ashley Davidoff MD TheCommonVein.net
45132 45133 45134 45135 45136 45139 45141
Lymphoma
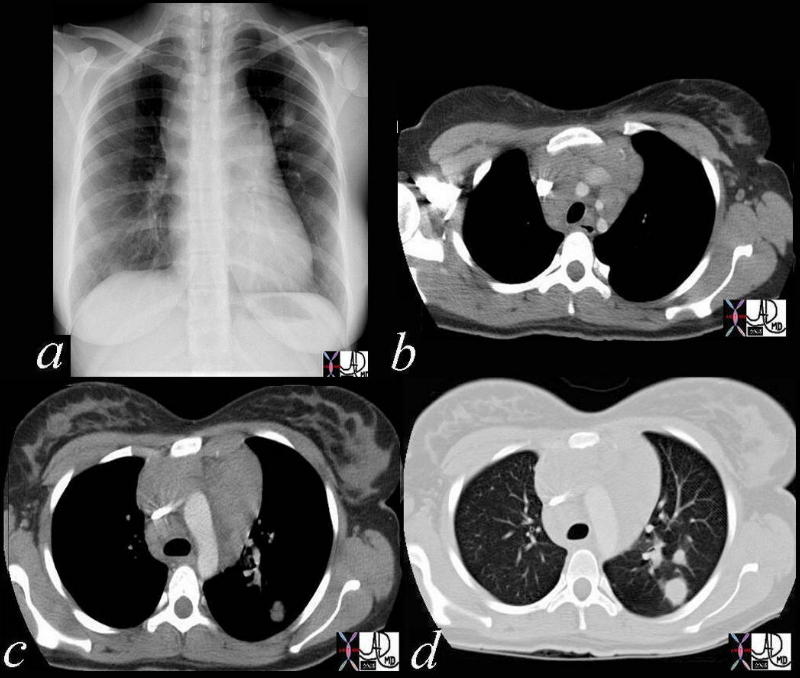

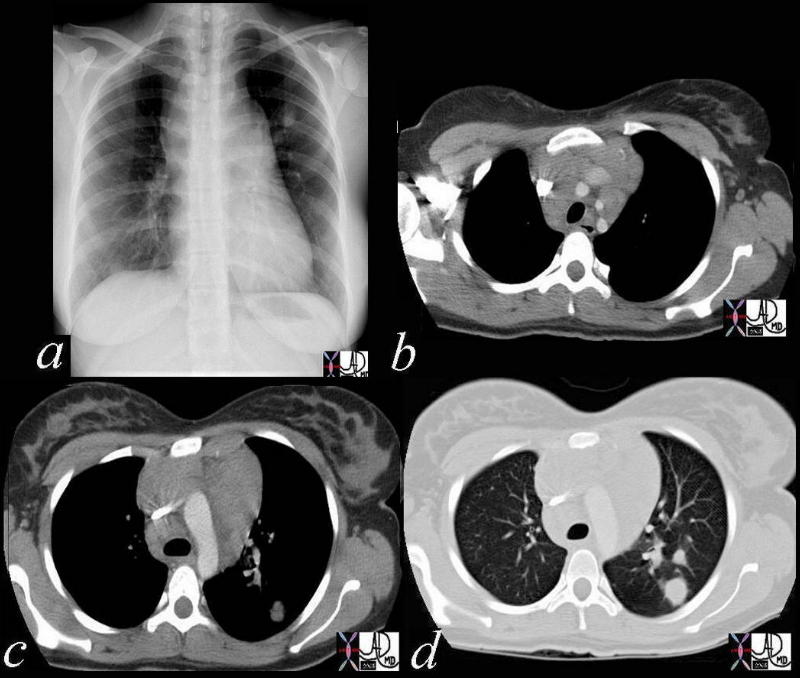
42068c03 Ashley Davidoff MD TheCommonVein.net medical students code chest enlarged lung lymph node lymphadenopathy lymphoma mediastinum nodule SVC compressed
Small cell Lung Carcinoma
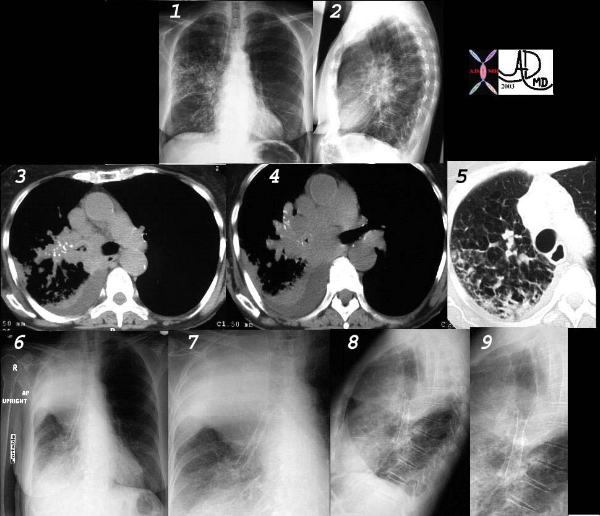

This collage of CT and plain film represents the radiological history of a patient with poorly differentiated small cell carcinoma, with extensive parenchymal involvement of the RUL and RML, and of the airways of RML and RLL. Images 1 and 2 show an interstitial and pneumonic pattern which was persistent over time. The CT shows extensive endobronchial disease involving right main stem (3,4) as well as almost all segments of RLL. This was confirmed by bronchoscopy.
Lymphangitic disease seems to be the dominant finding in the RUL on the lung windows. (5).
Following stent placement through a “pinhole” lesion, the patient occluded the RUL airways with tracheal shift and hyperinflation of the left lung (6,7,8,9). Clinically however, she improved greatly.
Courtesy Ashley Davidoff MD.
TheCommonVein.net
32426cl

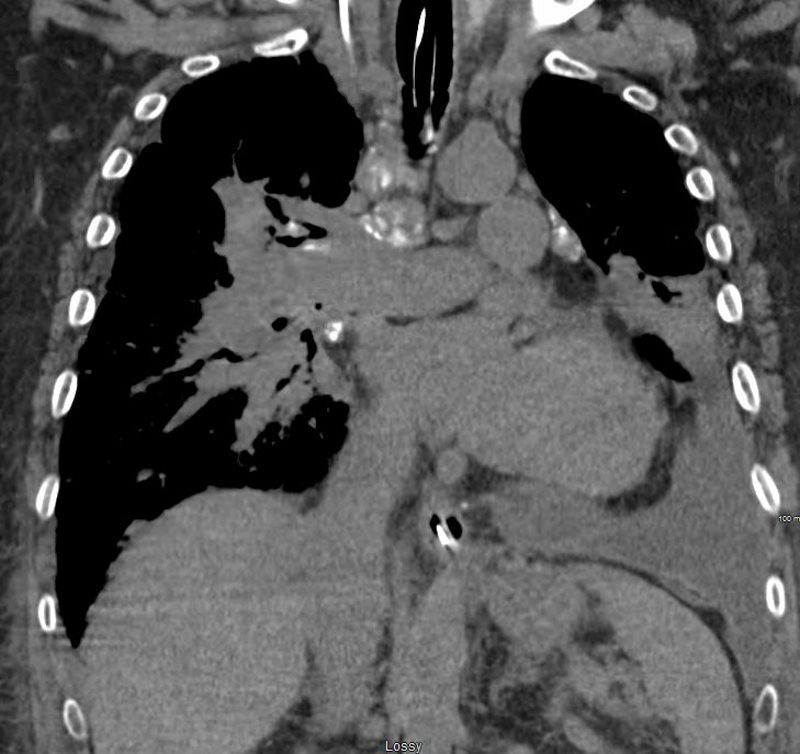
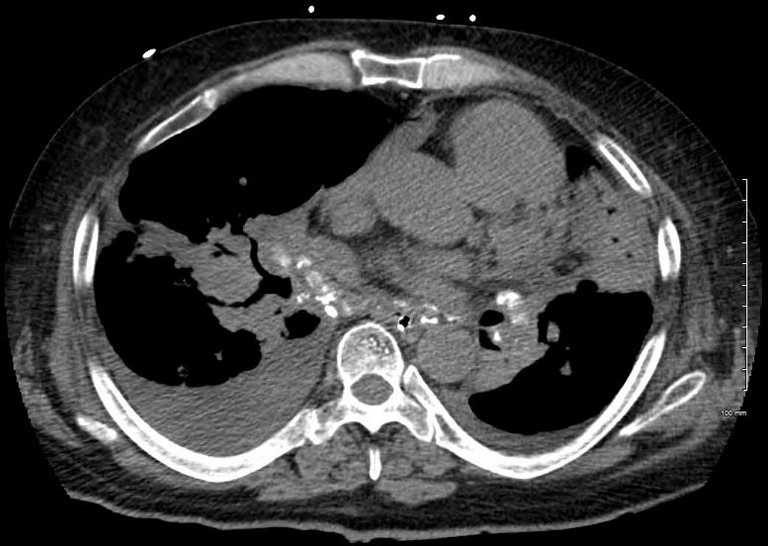
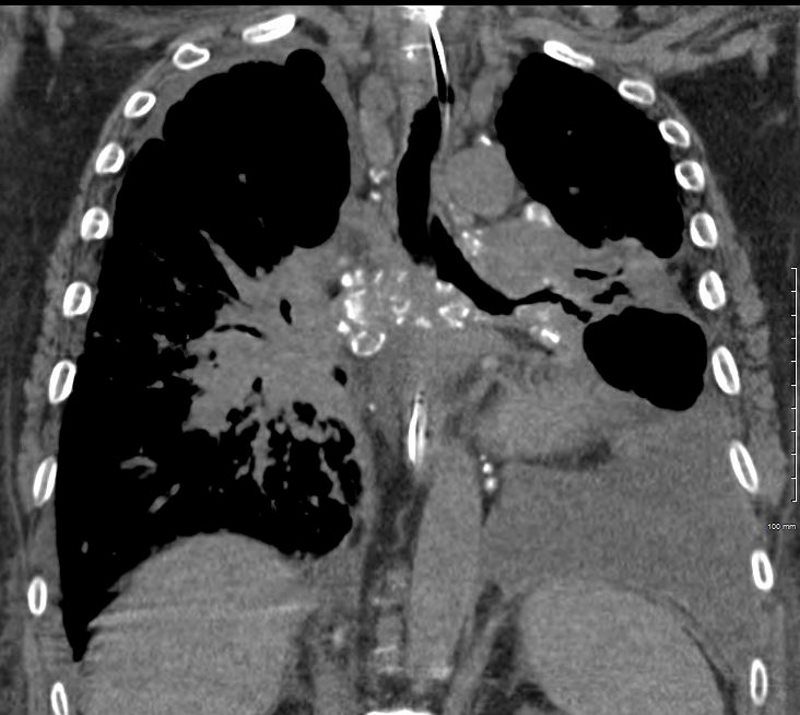
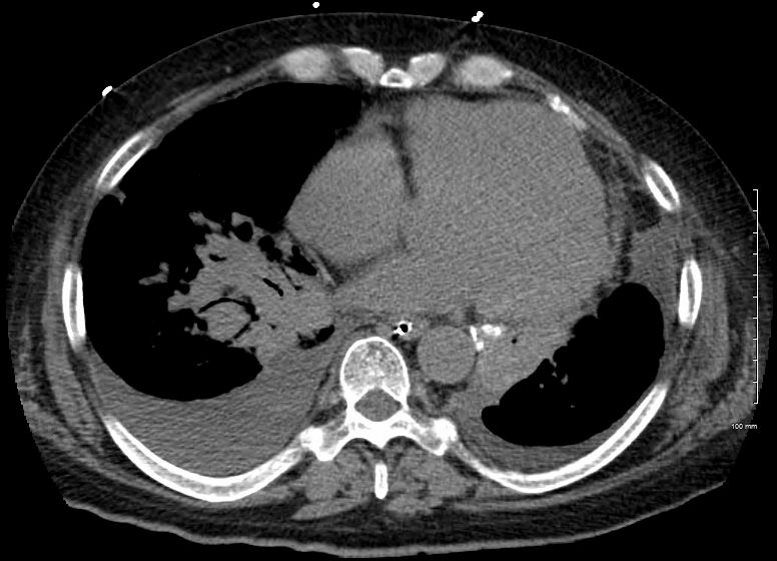
Elevated Right Main Stem Bronchus and Encasement
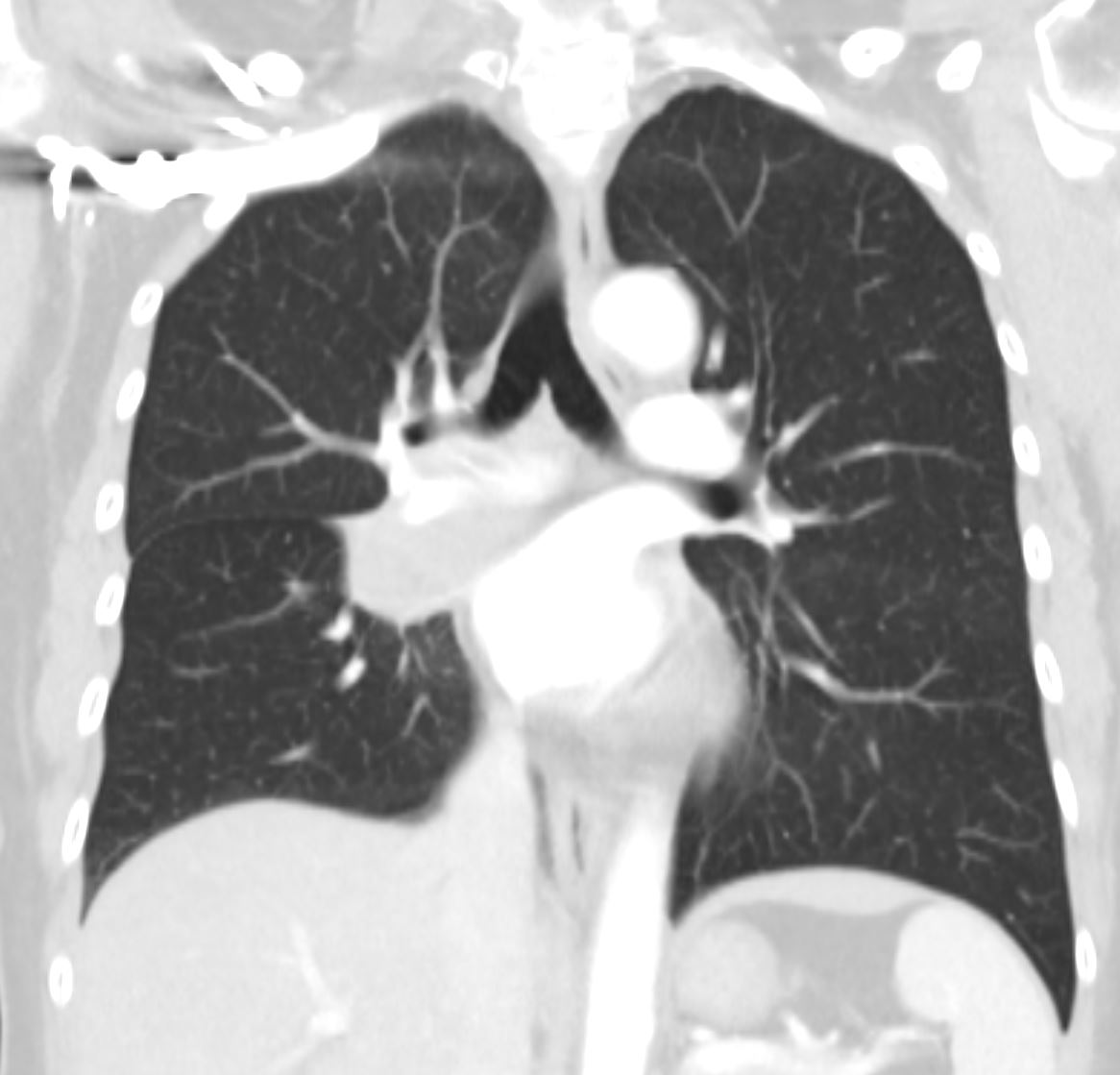

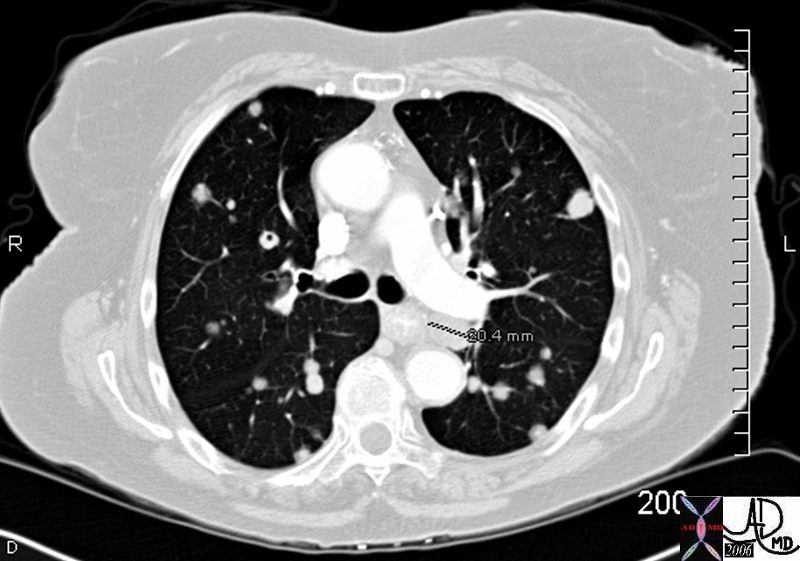
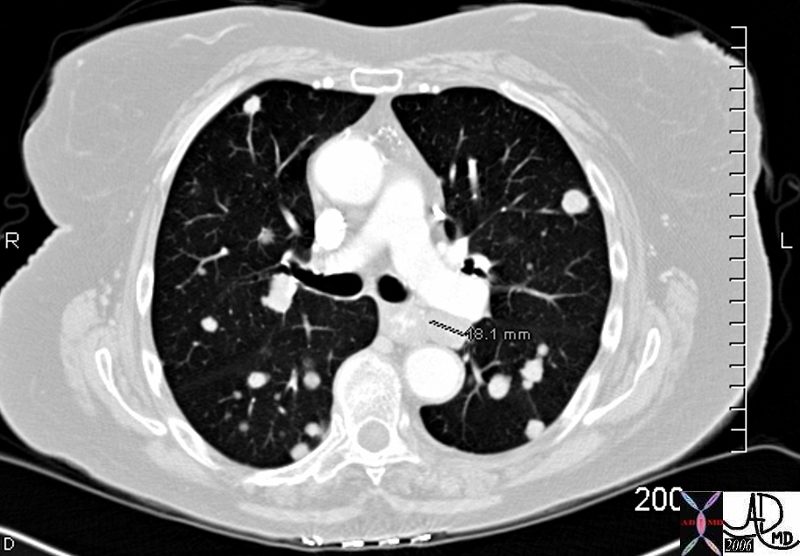
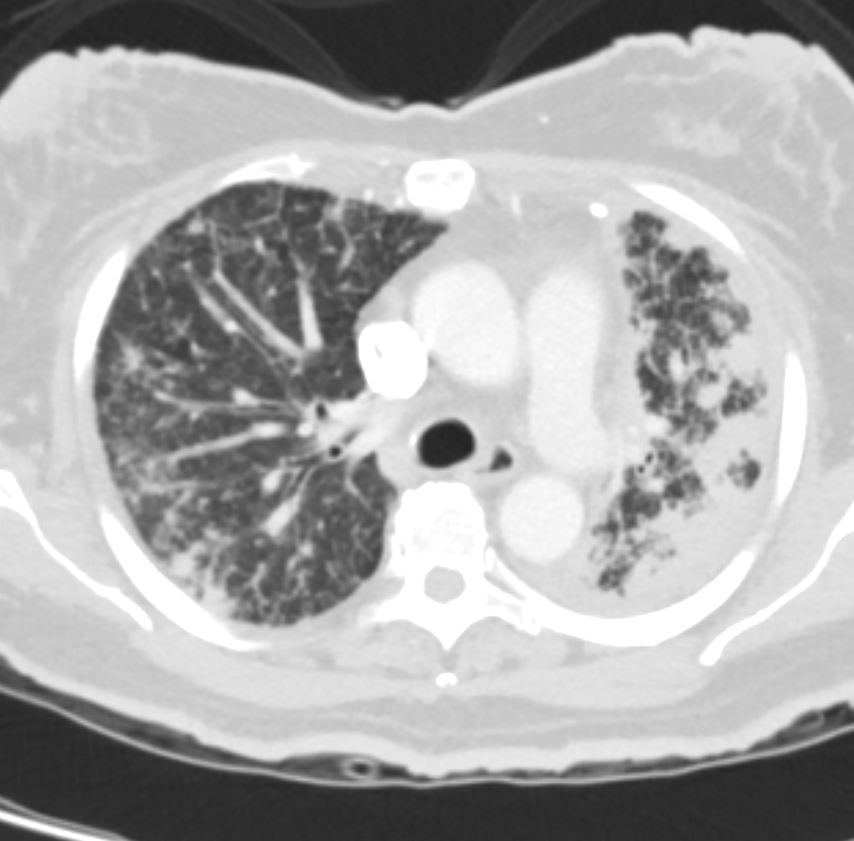
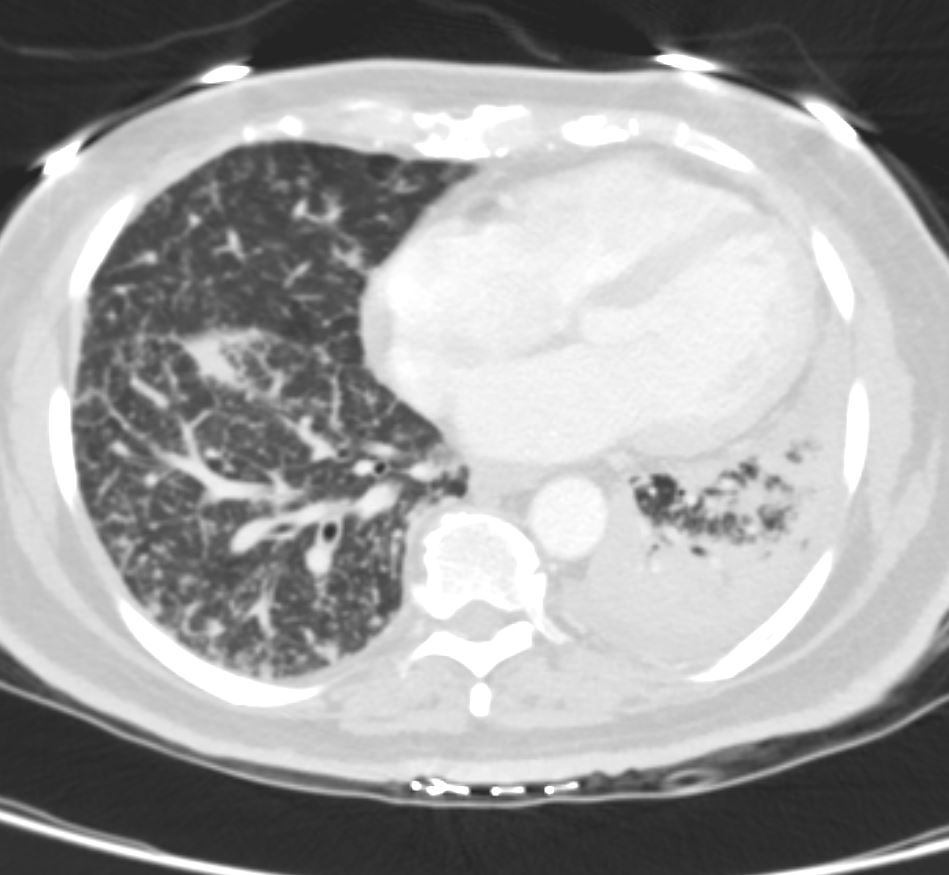
Bilateral Lymphangitis Carcinomatosis in a Patient with Adenocarcinoma


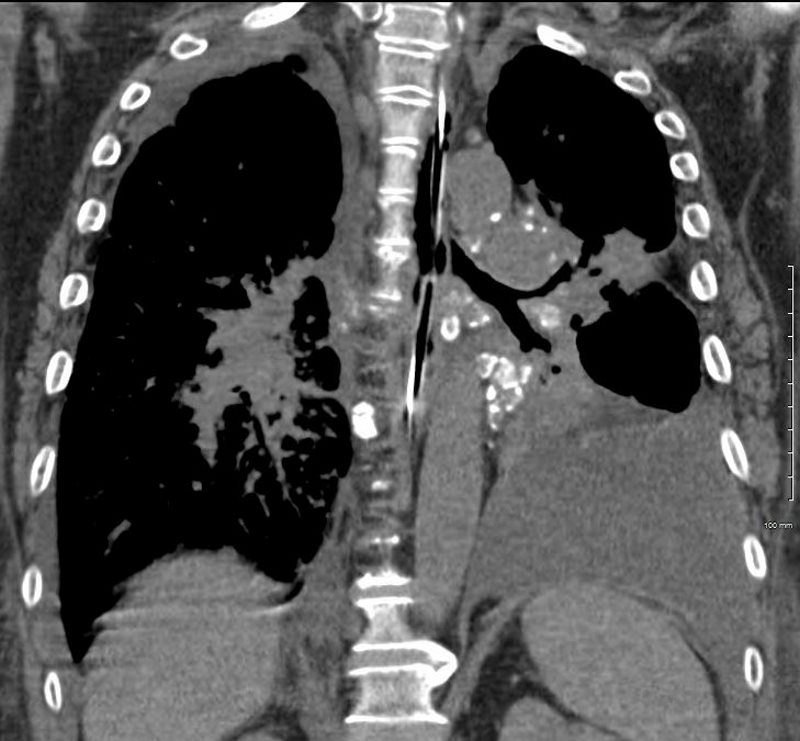
50 year old female with primary adenocarcinoma of the left lung with diffuse bilateral lymphangitic spread of disease characterized by lymphovascular distribution.
The nodularity on the fissures characterize the lymphatic distribution and the nodules are likely of a mixed nature, some being in the interlobular septa, and some in a centrilobular distribution .
Ashley Davidoff MD

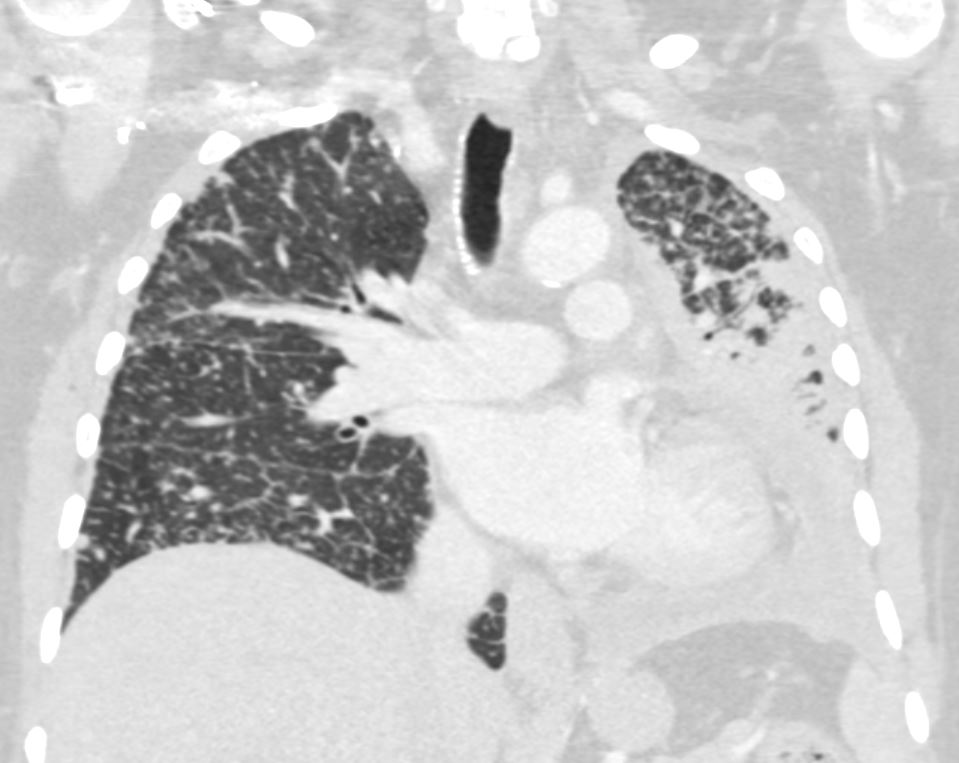
50 year old female with primary adenocarcinoma of the left lung with diffuse bilateral lymphangitic spread of disease characterized by lymphovascular distribution.
The nodularity on the fissures characterize the lymphatic distribution and the nodules are likely of a mixed nature, some being in the interlobular septa, and some in a centrilobular distribution .
Ashley Davidoff MD












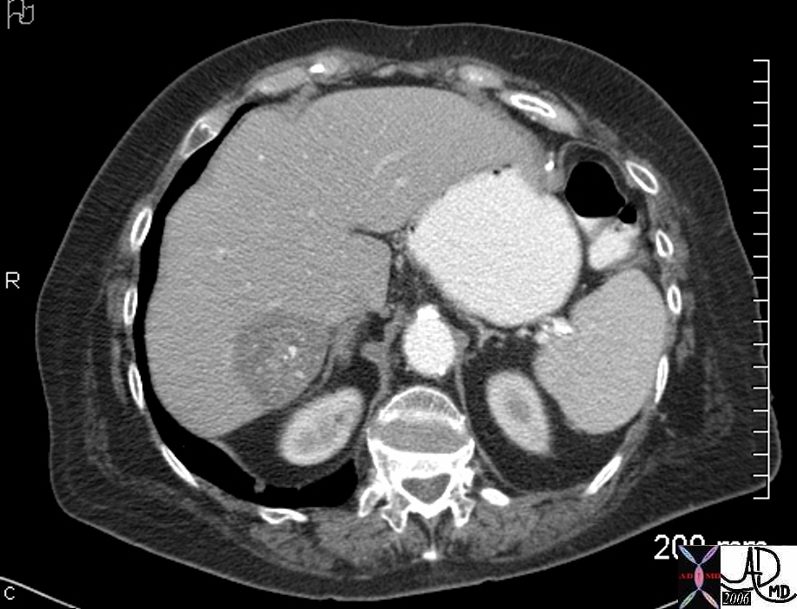
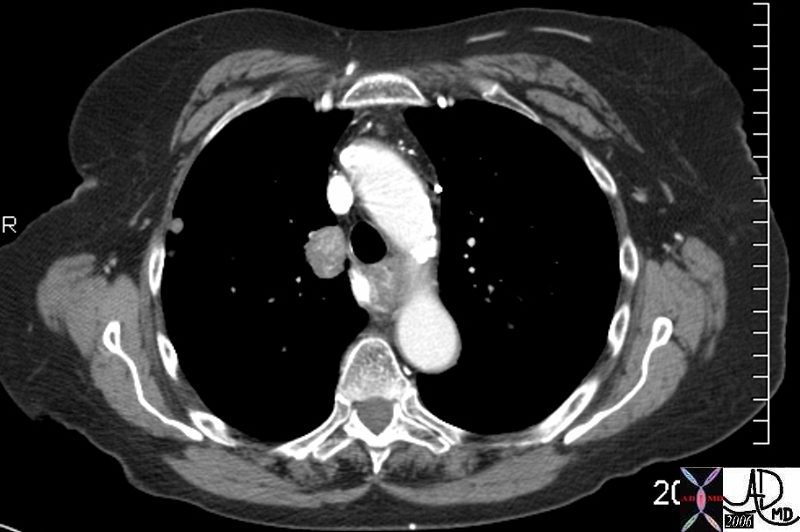
Lymphoma with Giant Rim Enhancing Lymph Nodes
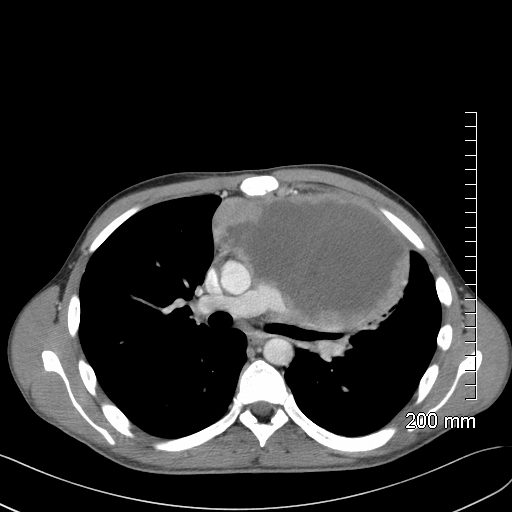

Ashley Davidoff MD TheCommonVein.net 118884
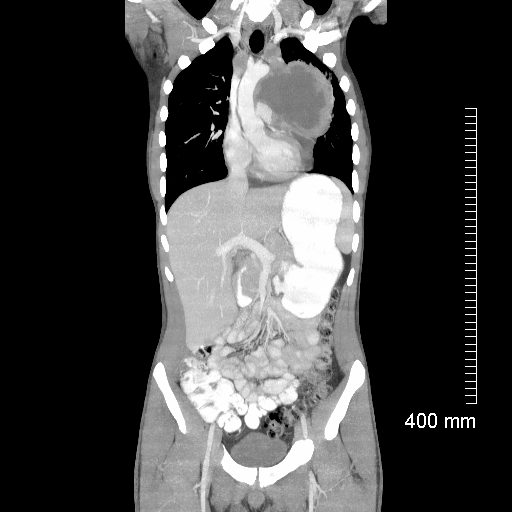

Ashley Davidoff MD TheCommonVein.net 118872
References and Links
Cases
044Lu Chronic Inactive TB Lymphatic Distribution
95Lu Sarcoidosis Lymph Nodes with Egg Shell Calcification